結腸炎研究的黃金標準。炎症性腸病是一種病因不明的多因素疾病,由兩種主要亞型組成,即潰瘍性結腸炎 (ulcerative colitis) 和克羅恩病 (Crohn’s disease)。硫酸葡聚醣 (Dextran sulfate sodium, DSS) 用於誘導動物的結腸炎的實驗。改變硫酸葡聚醣 (Dextran sulfate sodium, DSS) 的濃度或劑量的周期可以容易地誘發急性,慢性或複發性結腸炎。
什麼是硫酸葡聚醣 (Dextran sulfate sodium, DSS)?
硫酸葡聚醣 (Dextran sulfate sodium, DSS) 是帶負電荷的硫酸化葡聚醣,分子量為 40000Da。硫酸葡聚醣 (Dextran sulfate sodium, DSS) 廣泛用於在小鼠模型中誘導結腸炎。當硫酸葡聚醣 (Dextran sulfate sodium, DSS) 在飲用水中口服給藥時,誘發腸道炎症 (induce colitis)。使用 2%至 5%的濃度,在一周內出現症狀。
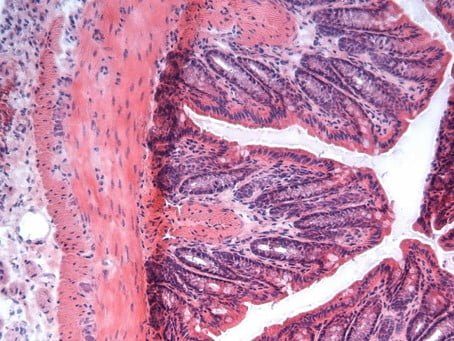
正常的老鼠腸組織
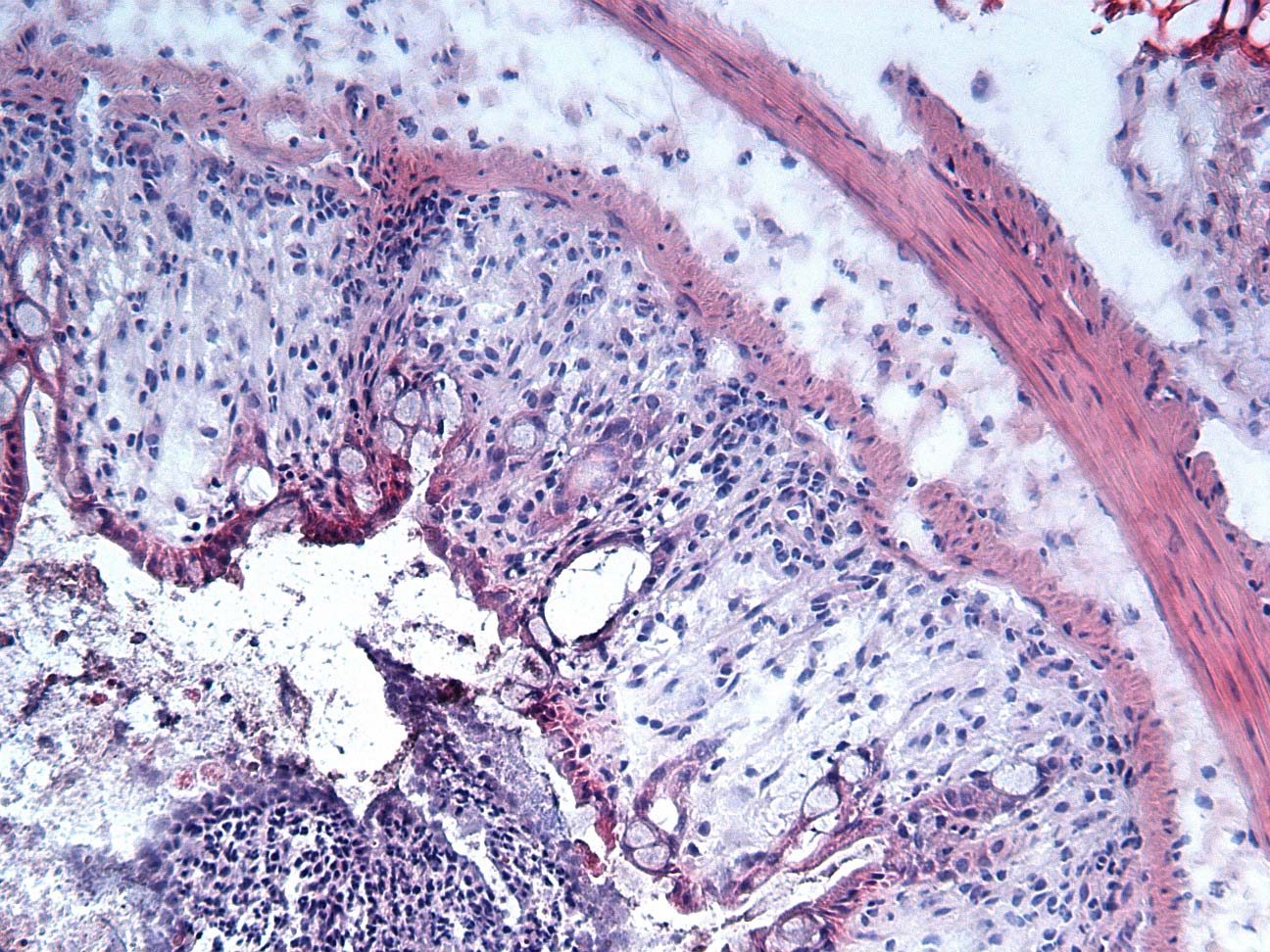
使用 DSS 誘導的腸組織使用 3% DSS 於飲用水中,作用 7 天。
如果您需要更大量的硫酸葡聚醣 (Dextran sulfate sodium, DSS),請立即聯絡我們取得報價。
參考文獻
2019-2017
- Wéra, O. et al. P2X1 ion channel deficiency causes massive bleeding in inflamed intestine and increases thrombosis. J. Thromb. Haemost (2019).
- Volk, J. K. et al. The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function. Journal of Experimental Medicine jem.20190679 (2019). doi:10.1084/jem.20190679
- Staats, S. et al. Dietary ursolic acid improves health span and life span in male Drosophila melanogaster. BioFactors 45, 169–186 (2019).
- Singh, K. et al. Dietary Arginine Regulates Severity of Experimental Colitis and Affects the Colonic Microbiome. Front. Cell. Infect. Microbiol. 9, (2019).
- Samba-Mondonga, M., Constante, M., Fragoso, G., Calvé, A. & Santos, M. M. Curcumin induces mild anemia in a DSS-induced colitis mouse model maintained on an iron-sufficient diet. PLOS ONE 14, e0208677 (2019).
- Salmenkari, H. et al. The use of unlicensed bone marrow–derived platelet lysate–expanded mesenchymal stromal cells in colitis: a pre-clinical study. Cytotherapy 21, 175–188 (2019).
- Polari, L. et al. Novel Selective Estrogen Receptor Modulator Ameliorates Murine Colitis. International Journal of Molecular Sciences 20, 3007 (2019).
- Neil, J. A. et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nature Microbiology 1 (2019). doi:10.1038/s41564-019-0470-1
- Markovic, M. et al. Phospholipid-Based Prodrugs for Colon-Targeted Drug Delivery: Experimental Study and In-Silico Simulations. Pharmaceutics 11, 186 (2019).
- Leleu-Chavain, N. et al. Benzo[d]thiazol-2(3H)-ones as new potent selective CB2 agonists with anti-inflammatory properties. European Journal of Medicinal Chemistry 165, 347–362 (2019).
- Jofra, T. et al. Experimental colitis in IL-10-deficient mice ameliorates in the absence of PTPN22. Clin. Exp. Immunol. 197, 263–275 (2019).
- Guillemot-Legris, O. et al. Colitis Alters Oxysterol Metabolism and is Affected by 4β-Hydroxycholesterol Administration. J Crohns Colitis 13, 218–229 (2019).
- Gowrikumar, S. et al. Upregulated claudin-1 expression promotes colitis-associated cancer by promoting β-catenin phosphorylation and activation in Notch/p-AKT-dependent manner. Oncogene 38, 5321 (2019).
- Garibay, D. et al. TGR5 Protects Against Colitis in Mice, but Vertical Sleeve Gastrectomy Increases Colitis Severity. OBES SURG 29, 1593–1601 (2019).
- Friedrich, M. et al. HDAC inhibitors promote intestinal epithelial regeneration via autocrine TGFβ1 signalling in inflammation. Mucosal Immunology 12, 656 (2019).
- Durmus, S. et al. ABC transporters Mdr1a/1b, Bcrp1, Mrp2 and Mrp3 determine the sensitivity to PhIP/DSS-induced colon carcinogenesis and inflammation. Arch Toxicol 93, 775–790 (2019).
- Dempsey, E., Abautret-Daly, Á., Docherty, N. G., Medina, C. & Harkin, A. Persistent central inflammation and region specific cellular activation accompany depression- and anxiety-like behaviours during the resolution phase of experimental colitis. Brain, Behavior, and Immunity (2019). doi:10.1016/j.bbi.2019.05.007
- De Vries, L. C. S. et al. A JAK1 Selective Kinase Inhibitor and Tofacitinib Affect Macrophage Activation and Function. Inflamm Bowel Dis 25, 647–660 (2019).
- Cribiù, F. M. et al. Using Robotic Systems to Process and Embed Colonic Murine Samples for Histological Analyses. JoVE (Journal of Visualized Experiments) e58654 (2019). doi:10.3791/58654
- Coburn, L. A. et al. Loss of solute carrier family 7 member 2 exacerbates inflammation-associated colon tumorigenesis. Oncogene 38, 1067 (2019).
- Chen, Y., Zhang, M. & Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 24, 513 (2019).
- Chang, Y.-L. et al. Therapeutic effects of a single injection of human umbilical mesenchymal stem cells on acute and chronic colitis in mice. Scientific Reports 9, 5832 (2019).
- Chang, C.-L. et al. Synergistic effect of combined melatonin and adipose-derived mesenchymal stem cell (ADMSC)-derived exosomes on amelioration of dextran sulfate sodium (DSS)-induced acute colitis. Am J Transl Res 11, 2706–2724 (2019).
- Casado‐Bedmar, M., Heil, S. D. S., Myrelid, P., Söderholm, J. D. & Keita, Å. V. Upregulation of intestinal mucosal mast cells expressing VPAC1 in close proximity to vasoactive intestinal polypeptide in inflammatory bowel disease and murine colitis. Neurogastroenterology & Motility 31, e13503 (2019).
- Burrello, C. et al. Mucosa-associated microbiota drives pathogenic functions in IBD-derived intestinal iNKT cells. Life Science Alliance 2, e201800229 (2019).
- Burrello, C. et al. Fecal Microbiota Transplantation Controls Murine Chronic Intestinal Inflammation by Modulating Immune Cell Functions and Gut Microbiota Composition. Cells 8, 517 (2019).
- Body-Malapel, M. et al. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for Inflammatory Bowel Diseases. Mucosal Immunology 12, 468 (2019).
- Al-Omari, M. M., Razan B. Al-Ghariebeh, A. A. A. A., Zoubi, H. A.- & Al-Qaoud, K. M. Camel milk Whey Inhibits Inflammatory Colorectal Cancer Development Via Down regulation of Pro-inflammatory Cytokines in Induced AOM/DSS Mouse Model. 1 256–262 (2019). doi:10.9755/ejfa.2019.v31.i4.1935
- Aden, K. et al. Epithelial RNase H2 Maintains Genome Integrity and Prevents Intestinal Tumorigenesis in Mice. Gastroenterology 156, 145-159.e19 (2019).
- Willemze, R. A. et al. Neuronal control of experimental colitis occurs via sympathetic intestinal innervation. Neurogastroenterology & Motility 30, e13163 (2018).
- Singh, A. K., Hertzberger, R. Y. & Knaus, U. G. Hydrogen peroxide production by lactobacilli promotes epithelial restitution during colitis. Redox Biology 16, 11–20 (2018).
- Sferra, R. et al. Interaction between sphingosine kinase/sphingosine 1 phosphate and transforming growth factor-β/Smads pathways in experimental intestinal fibrosis. An in vivo immunohistochemical study. Eur J Histochem 62, (2018).
- Salmenkari, H., Pasanen, L., Linden, J., Korpela, R. & Vapaatalo, H. Beneficial anti-inflammatory effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in the treatment of dextran sulfate sodium-induced colitis in mice. J. Physiol. Pharmacol. 69, (2018).
- Radulovic, K. et al. A dietary flavone confers communicable protection against colitis through NLRP6 signaling independently of inflammasome activation. Mucosal Immunol 11, 811–819 (2018).
- Paveljšek, D. et al. Lactobacillus fermentum L930BB and Bifidobacterium animalis subsp. animalis IM386 initiate signalling pathways involved in intestinal epithelial barrier protection. Beneficial Microbes 9, 515–525 (2018).
- Nikolic, A. et al. Intraperitoneal administration of mesenchymal stem cells ameliorates acute dextran sulfate sodium-induced colitis by suppressing dendritic cells. Biomedicine & Pharmacotherapy 100, 426–432 (2018).
- Messal, N. et al. Ectopic expression of OX1R in ulcerative colitis mediates anti-inflammatory effect of orexin-A. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 1864, 3618–3628 (2018).
- Masquelier, J. et al. Lysophosphatidylinositols in inflammation and macrophage activation: Altered levels and anti-inflammatory effects. Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids 1863, 1458–1468 (2018).
- Liu, X. et al. 1-L-MT, an IDO inhibitor, prevented colitis-associated cancer by inducing CDC20 inhibition-mediated mitotic death of colon cancer cells. International Journal of Cancer 143, 1516–1529 (2018).
- Koren, E. et al. ARTS mediates apoptosis and regeneration of the intestinal stem cell niche. Nature Communications 9, 4582 (2018).
- Kesharwani, S. S. et al. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. Journal of Controlled Release 290, 165–179 (2018).
- K. Martin, P. et al. Autophagy proteins suppress protective type I interferon signaling in response to the murine gut microbiota. Nature Microbiology 3, (2018).
- Greicius, G. et al. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. PNAS 115, E3173–E3181 (2018).
- Gobert, A. P. et al. Distinct Immunomodulatory Effects of Spermine Oxidase in Colitis Induced by Epithelial Injury or Infection. Front. Immunol. 9, (2018).
- Farombi, E. O. et al. 6-Gingerol improves testicular function in mice model of chronic ulcerative colitis. Hum Exp Toxicol 37, 358–372 (2018).
- Fan, T.-J. et al. Environmental Factors Modify the Severity of Acute DSS Colitis in Caspase-11-Deficient Mice. Inflamm Bowel Dis 24, 2394–2403 (2018).
- Ducheix, S. et al. Deletion of Stearoyl-CoA Desaturase-1 From the Intestinal Epithelium Promotes Inflammation and Tumorigenesis, Reversed by Dietary Oleate. Gastroenterology 155, 1524-1538.e9 (2018).
- Darnaud, M. et al. Enteric Delivery of Regenerating Family Member 3 alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice With Colitis. Gastroenterology 154, 1009-1023.e14 (2018).
- Da Silva, S. et al. A Novel Topical PPARγ Agonist Induces PPARγ Activity in Ulcerative Colitis Mucosa and Prevents and Reverses Inflammation in Induced Colitis Models. Inflamm Bowel Dis 24, 792–805 (2018).
- Cuellar-Nuñez, M. L. et al. Physicochemical and nutraceutical properties of moringa (Moringa oleifera) leaves and their effects in an in vivo AOM/DSS-induced colorectal carcinogenesis model. Food Research International 105, 159–168 (2018).
- Cribiù, F. M. et al. Implementation of an automated inclusion system for the histological analysis of murine tissue samples: A feasibility study in DSS-induced chronic colitis. Eur J Inflamm 16, 2058739218776883 (2018).
- Cardoso, A. et al. The Dynamics of Interleukin-10-Afforded Protection during Dextran Sulfate Sodium-Induced Colitis. Front. Immunol. 9, (2018).
- Burrello, C. et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nature Communications 9, 5184 (2018).
- Burrello, C. et al. Short-term Oral Antibiotics Treatment Promotes Inflammatory Activation of Colonic Invariant Natural Killer T and Conventional CD4+ T Cells. Front. Med. 5, (2018).
- Alhouayek, M., Buisseret, B., Paquot, A., Guillemot-Legris, O. & Muccioli, G. G. The endogenous bioactive lipid prostaglandin D2-glycerol ester reduces murine colitis via DP1 and PPARγ receptors. The FASEB Journal 32, 5000–5011 (2018).
- Ajayi, B., Adedara, I. & Farombi, E. Protective mechanisms of 6-gingerol in dextran sulfate sodium-induced chronic ulcerative colitis in mice. Hum Exp Toxicol 37, 1054–1068 (2018).
- Ajayi, B. O., Adedara, I. A., Ajani, O. S., Oyeyemi, M. O. & Farombi, E. O. [6]-Gingerol modulates spermatotoxicity associated with ulcerative colitis and benzo[a]pyrene exposure in BALB/c mice. Journal of Basic and Clinical Physiology and Pharmacology 29, 247–256 (2018).
- Acovic, A. et al. Indoleamine 2,3-dioxygenase-dependent expansion of T-regulatory cells maintains mucosal healing in ulcerative colitis. Therap Adv Gastroenterol 11, 1756284818793558 (2018).
- Zhdanov, A. V. et al. Quantitative analysis of mucosal oxygenation using ex vivo imaging of healthy and inflamed mammalian colon tissue. Cell. Mol. Life Sci. 74, 141–151 (2017).
- Udden, S. M. N. et al. NOD2 Suppresses Colorectal Tumorigenesis via Downregulation of the TLR Pathways. Cell Reports 19, 2756–2770 (2017).
- Tubbs, A. L., Liu, B., Rogers, T. D., Sartor, R. B. & Miao, E. A. Dietary Salt Exacerbates Experimental Colitis. The Journal of Immunology 199, 1051–1059 (2017).
- Sünderhauf, A. et al. Regulation of epithelial cell expressed C3 in the intestine – Relevance for the pathophysiology of inflammatory bowel disease? Molecular Immunology 90, 227–238 (2017).
- Štofilová, J. et al. Cytokine production in vitro and in rat model of colitis in response to Lactobacillus plantarum LS/07. Biomedicine & Pharmacotherapy 94, 1176–1185 (2017).
- Smole, A., Lainšček, D., Bezeljak, U., Horvat, S. & Jerala, R. A Synthetic Mammalian Therapeutic Gene Circuit for Sensing and Suppressing Inflammation. Molecular Therapy 25, 102–119 (2017).
- Pagel, R. et al. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. The FASEB Journal 31, 4707–4719 (2017).
- O’Sullivan, S. et al. Inhibition of matrix metalloproteinase-9 by a barbiturate-nitrate hybrid ameliorates dextran sulphate sodium-induced colitis: effect on inflammation-related genes: Nitrate inhibition of MMP-9 in DSS-induced colitis. British Journal of Pharmacology 174, 512–524 (2017).
- Menghini, P. et al. A novel model of colitis-associated cancer in SAMP1/YitFc mice with Crohn’s disease-like ileitis. PLoS ONE 12, e0174121 (2017).
- Matsuzawa-Ishimoto, Y. et al. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. Journal of Experimental Medicine 214, 3687–3705 (2017).
- Martin, A., Emmenegger, S., Hinkelmann, K. & Thönssen, B. A viewpoint-based case-based reasoning approach utilising an enterprise architecture ontology for experience management. Enterprise Information Systems 11, 551–575 (2017).
- Markovic, B. S. et al. Bacterial Flora Play Important Roles in Acute Dextran Sulphate Sodium-Induced Colitis But Are Not Involved in Gal-3 Dependent Modulation of Colon Inflammation. Serbian Journal of Experimental and Clinical Research 18, 213–220 (2017).
- Luna-Vital, D. A., González de Mejía, E. & Loarca-Piña, G. Dietary Peptides from Phaseolus vulgaris L. Reduced AOM/DSS-Induced Colitis-Associated Colon Carcinogenesis in Balb/c Mice. Plant Foods Hum Nutr 72, 445–447 (2017).
- Li, X. et al. Myeloid-derived cullin 3 promotes STAT3 phosphorylation by inhibiting OGT expression and protects against intestinal inflammation. Journal of Experimental Medicine 214, 1093–1109 (2017).
- Khelifi, L., Soufli, I., Labsi, M. & Touil-Boukoffa, C. Immune-protective effect of echinococcosis on colitis experimental model is dependent of down regulation of TNF-α and NO production. Acta Tropica 166, 7–15 (2017).
- Katlinski, K. V. et al. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer Cell 31, 194–207 (2017).
- Hardbower, D. M. et al. EGFR-mediated macrophage activation promotes colitis-associated tumorigenesis. Oncogene 36, 3807–3819 (2017).
- Gregorio, J. D. et al. Role of glycogen synthase kinase-3β and PPAR-γ on epithelial-to-mesenchymal transition in DSS-induced colorectal fibrosis. PLOS ONE 12, e0171093 (2017).
- Fugmann, T., Sofron, A., Ritz, D., Bootz, F. & Neri, D. The MHC Class II Immunopeptidome of Lymph Nodes in Health and in Chemically Induced Colitis. J. Immunol. 198, 1357–1364 (2017).
- Fehér, Á. et al. Analysing the effect of I1 imidazoline receptor ligands on DSS-induced acute colitis in mice. Inflammopharmacol 25, 107–118 (2017).
- El‑Salhy, M., Umezawa, K., Hatlebakk, J. G. & Gilja, O. H. Abnormal differentiation of stem cells into enteroendocrine cells in rats with DSS-induced colitis. Molecular Medicine Reports 15, 2106–2112 (2017).
- El‑Salhy, M., Hatlebakk, J. G. & Gilja, O. H. Abnormalities in endocrine and immune cells are correlated in dextran‑sulfate‑sodium‑induced colitis in rats. Molecular Medicine Reports 15, 12–20 (2017).
- Do, A. et al. An HDAC6 Inhibitor Confers Protection and Selectively Inhibits B-Cell Infiltration in DSS-Induced Colitis in Mice. J Pharmacol Exp Ther 360, 140–151 (2017).
- Constante, M., Fragoso, G., Lupien-Meilleur, J., Calvé, A. & Santos, M. M. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm Bowel Dis 23, 753–766 (2017).
- Constante, M., Fragoso, G., Calvé, A., Samba-Mondonga, M. & Santos, M. M. Dietary Heme Induces Gut Dysbiosis, Aggravates Colitis, and Potentiates the Development of Adenomas in Mice. Front. Microbiol. 8, (2017).
- Carvajal, A. E. et al. Reelin expression is up-regulated in mice colon in response to acute colitis and provides resistance against colitis. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 1863, 462–473 (2017).
- Carvajal, A. E. et al. Reelin protects from colon pathology by maintaining the intestinal barrier integrity and repressing tumorigenic genes. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 1863, 2126–2134 (2017).
- Berlec, A. et al. Dextran sulphate sodium colitis in C57BL/6J mice is alleviated by Lactococcus lactis and worsened by the neutralization of Tumor necrosis Factor α. International Immunopharmacology 43, 219–226 (2017).
- Adedara, I. A., Ajayi, B. O., Awogbindin, I. O. & Farombi, E. O. Interactive effects of ethanol on ulcerative colitis and its associated testicular dysfunction in pubertal BALB/c mice. Alcohol 64, 65–75 (2017).
2016-2012
- Yu, C. et al. Platelet-Derived CCL5 Regulates CXC Chemokine Formation and Neutrophil Recruitment in Acute Experimental Colitis. J. Cell. Physiol. 231, 370–376 (2016).
- Vázquez-Carretero, M. D. et al. The Synaptojanins in the murine small and large intestine. J Bioenerg Biomembr 48, 569–579 (2016).
- Speca, S. et al. Novel PPARγ Modulator GED-0507-34 Levo Ameliorates Inflammation-driven Intestinal Fibrosis. Inflamm Bowel Dis 22, 279–292 (2016).
- Simovic Markovic, B. et al. Pharmacological Inhibition of Gal-3 in Mesenchymal Stem Cells Enhances Their Capacity to Promote Alternative Activation of Macrophages in Dextran Sulphate Sodium-Induced Colitis. Stem Cells International (2016). doi:10.1155/2016/2640746
- Simovic Markovic, B. et al. Galectin-3 Plays an Important Pro-inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages. J Crohns Colitis 10, 593–606 (2016).
- Shen, F. et al. Vinegar Treatment Prevents the Development of Murine Experimental Colitis via Inhibition of Inflammation and Apoptosis. J. Agric. Food Chem. 64, 1111–1121 (2016).
- Ohta, T. et al. Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Scientific Reports 6, 23505 (2016).
- O’Shea, C. J. et al. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. Journal of Nutritional Science 5, (2016).
- Misiorek, J. O. et al. Keratin 8-deletion induced colitis predisposes to murine colorectal cancer enforced by the inflammasome and IL-22 pathway. Carcinogenesis 37, 777–786 (2016).
- Matthis, A. L. et al. Importance of the Evaluation of N-Acetyltransferase Enzyme Activity Prior to 5-Aminosalicylic Acid Medication for Ulcerative Colitis. Inflamm Bowel Dis 22, 1793–1802 (2016).
- Martin, J. C. et al. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunology 9, 539–549 (2016).
- Márquez-Flores, Y. K., Villegas, I., Cárdeno, A., Rosillo, M. Á. & Alarcón-de-la-Lastra, C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. The Journal of Nutritional Biochemistry 30, 143–152 (2016).
- Lee, S. et al. Arhgap17, a RhoGTPase activating protein, regulates mucosal and epithelial barrier function in the mouse colon. Scientific Reports 6, 26923 (2016).
- Kriščiukaitis, A. et al. Elaboration of Optimized Expert Knowledge Based Quantitative Features for Automatic Histological Image Evaluation. BIOMEDICAL ENGINEERING 2016 19, (2016).
- Jia, L.-G. et al. A Novel Role for TL1A/DR3 in Protection against Intestinal Injury and Infection. J. Immunol. 197, 377–386 (2016).
- Helenius, T. O., Antman, C. A., Asghar, M. N., Nyström, J. H. & Toivola, D. M. Keratins Are Altered in Intestinal Disease-Related Stress Responses. Cells 5, 35 (2016).
- Helenius, T. Structure in stress management : Keratins in intestinal stress protection. (2016).
- Heinsbroek, S. E. M. et al. miR-511-3p, embedded in the macrophage mannose receptor gene, contributes to intestinal inflammation. Mucosal Immunology 9, 960–973 (2016).
- Guada, M. et al. Cyclosporine A-loaded lipid nanoparticles in inflammatory bowel disease. International Journal of Pharmaceutics 503, 196–198 (2016).
- Goodman, W. A. et al. KLF6 contributes to myeloid cell plasticity in the pathogenesis of intestinal inflammation. Mucosal Immunology 9, 1250–1262 (2016).
- Gerling, M. et al. Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nature Communications 7, 12321 (2016).
- Farombi, E. O. et al. Dietary protocatechuic acid ameliorates dextran sulphate sodium-induced ulcerative colitis and hepatotoxicity in rats. Food Funct. 7, 913–921 (2016).
- Elmasry, A., Daba, M.-H. & El-Karef, A. Possible Effects of Moringa oleifera versus Ginger (Zingiber officinalis) on Experimental Colitis in Mice. BJMMR 16, 1–19 (2016).
- El-Salhy, M. & Umezawa, K. Treatment with novel AP-1 and NF-κB inhibitors restores the colonic endocrine cells to normal levels in rats with DSS-induced colitis. International Journal of Molecular Medicine 37, 556–564 (2016).
- El-Salhy, M. & Umezawa, K. Anti-inflammatory effects of novel AP-1 and NF-κB inhibitors in dextran-sulfate-sodium-induced colitis in rats. International Journal of Molecular Medicine 37, 1457–1464 (2016).
- Däbritz, J., Judd, L. M., Chalinor, H. V., Menheniott, T. R. & Giraud, A. S. Altered gp130 signalling ameliorates experimental colitis via myeloid cell-specific STAT3 activation and myeloid-derived suppressor cells. Sci Rep 6, 20584 (2016).
- Dolowschiak, T. et al. IFN-γ Hinders Recovery from Mucosal Inflammation during Antibiotic Therapy for Salmonella Gut Infection. Cell Host & Microbe 20, 238–249 (2016).
- Di Martino, L. et al. Protective Role for TWEAK/Fn14 in Regulating Acute Intestinal Inflammation and Colitis-Associated Tumorigenesis. Cancer Res. 76, 6533–6542 (2016).
- Di Giovangiulio, M. et al. Vagotomy Affects the Development of Oral Tolerance and Increases Susceptibility to Develop Colitis Independently of α-7 Nicotinic Receptor. Mol Med 22, 464–476 (2016).
- De Fazio, L. et al. Dietary Geraniol by Oral or Enema Administration Strongly Reduces Dysbiosis and Systemic Inflammation in Dextran Sulfate Sodium-Treated Mice. Front. Pharmacol. 7, (2016).
- Das, S. et al. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene 35, 2645–2654 (2016).
- Chng, S. H. et al. Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity. Scientific Reports 6, 23820 (2016).
- Brauer, R. et al. MMP-19 deficiency causes aggravation of colitis due to defects in innate immune cell function. Mucosal Immunology 9, 974–985 (2016).
- Bosma, M. et al. FNDC4 acts as an anti-inflammatory factor on macrophages and improves colitis in mice. Nature Communications 7, 11314 (2016).
- Bootz, F., Ziffels, B. & Neri, D. Antibody-Based Targeted Delivery of Interleukin-22 Promotes Rapid Clinical Recovery in Mice With DSS-Induced Colitis. Inflamm Bowel Dis 22, 2098–2105 (2016).
- Beloqui, A. et al. A comparative study of curcumin-loaded lipid-based nanocarriers in the treatment of inflammatory bowel disease. Colloids and Surfaces B: Biointerfaces 143, 327–335 (2016).
- Ahl, D. et al. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiologica 217, 300–310 (2016).
- Aden, K. et al. Epithelial IL-23R Signaling Licenses Protective IL-22 Responses in Intestinal Inflammation. Cell Reports 16, 2208–2218 (2016).
- Zwicker, S. et al. Interleukin 34: a new modulator of human and experimental inflammatory bowel disease. Clinical Science 129, 281–290 (2015).
- Zhdanov, A. V., Okkelman, I. A., Collins, F. W. J., Melgar, S. & Papkovsky, D. B. A novel effect of DMOG on cell metabolism: direct inhibition of mitochondrial function precedes HIF target gene expression. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1847, 1254–1266 (2015).
- Warfield, K. L. et al. A Novel Iminosugar UV-12 with Activity against the Diverse Viruses Influenza and Dengue (Novel Iminosugar Antiviral for Influenza and Dengue). Viruses 7, 2404–2427 (2015).
- Te Velde, A. A. et al. Effects of Dietary Plant Sterols and Stanol Esters with Low- and High-Fat Diets in Chronic and Acute Models for Experimental Colitis. Nutrients 7, 8518–8531 (2015).
- Talero, E. et al. Inhibition of chronic ulcerative colitis-associated adenocarcinoma development in mice by VSL#3. Inflamm. Bowel Dis. 21, 1027–1037 (2015).
- Spisni, E. et al. Cyclooxygenase-2 Silencing for the Treatment of Colitis: A Combined In Vivo Strategy Based on RNA Interference and Engineered Escherichia Coli. Molecular Therapy 23, 278–289 (2015).
- Soufli, I. et al. Crude extract of hydatid laminated layer from Echinococcus granulosus cyst attenuates mucosal intestinal damage and inflammatory responses in Dextran Sulfate Sodium induced colitis in mice. Journal of Inflammation 12, 19 (2015).
- Sommer, F. & Bäckhed, F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunology 8, 372–379 (2015).
- Petrolis, R. et al. Digital imaging of colon tissue: method for evaluation of inflammation severity by spatial frequency features of the histological images. Diagn Pathol 10, 159 (2015).
- Moon, C. et al. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 521, 90–93 (2015).
- Hu, S. et al. The DNA Sensor AIM2 Maintains Intestinal Homeostasis via Regulation of Epithelial Antimicrobial Host Defense. Cell Reports 13, 1922–1936 (2015).
- Helenius, T. O. et al. Keratin 8 absence down-regulates colonocyte HMGCS2 and modulates colonic ketogenesis and energy metabolism. Mol. Biol. Cell 26, 2298–2310 (2015).
- Heinsbroek, S. E. M. et al. Orally delivered β-glucans aggravate dextran sulfate sodium (DSS)-induced intestinal inflammation. Nutrition Research 35, 1106–1112 (2015).
- Gouyer, V. et al. Delivery of a mucin domain enriched in cysteine residues strengthens the intestinal mucous barrier. Scientific Reports 5, 9577 (2015).
- Forte, D. et al. Human cord blood-derived platelet lysate enhances the therapeutic activity of adipose-derived mesenchymal stromal cells isolated from Crohn’s disease patients in a mouse model of colitis. Stem Cell Research & Therapy 6, 170 (2015).
- Fornasa, G. et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. Journal of Allergy and Clinical Immunology 136, 413–422 (2015).
- Elkatary, R. et al. Effect of Different Doses of Sitagliptin in Treatment of Experimentally Induced Colitis in Mice. BJPR 7, 140–151 (2015).
- Bootz, F., Schmid, A. S. & Neri, D. Alternatively Spliced EDA Domain of Fibronectin Is a Target for Pharmacodelivery Applications in Inflammatory Bowel Disease. Inflamm Bowel Dis 21, 1908–1917 (2015).
- Banerjee, A. et al. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Research & Therapy 6, 79 (2015).
- Asghar, M. N. et al. The Amount of Keratins Matters for Stress Protection of the Colonic Epithelium. PLOS ONE 10, e0127436 (2015).
- Ajayi, B. O., Adedara, I. A. & Farombi, E. O. Pharmacological activity of 6-gingerol in dextran sulphate sodium-induced ulcerative colitis in BALB/c mice. Phytother Res 29, 566–572 (2015).
- Wang, S., Hibberd, M. L., Pettersson, S. & Lee, Y. K. Enterococcus faecalis from Healthy Infants Modulates Inflammation through MAPK Signaling Pathways. PLOS ONE 9, e97523 (2014).
- Sommer, F. et al. Altered Mucus Glycosylation in Core 1 O-Glycan-Deficient Mice Affects Microbiota Composition and Intestinal Architecture. PLOS ONE 9, e85254 (2014).
- Silva, Z. E. V. da, Lehr, H.-A. & Velin, D. In vitro and in vivo Repair Activities of Undifferentiated and Classically and Alternatively Activated Macrophages. PAT 81, 86–93 (2014).
- Maillard, M. H. et al. Toll-interacting Protein Modulates Colitis Susceptibility in Mice. Inflamm Bowel Dis 20, 660–670 (2014).
- Kernbauer, E., Ding, Y. & Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014).
- Judd, L. M. et al. TFF2 deficiency exacerbates weight loss and alters immune cell and cytokine profiles in DSS colitis, and this cannot be rescued by wild-type bone marrow. American Journal of Physiology-Gastrointestinal and Liver Physiology 308, G12–G24 (2014).
- Jakobsson, T. et al. The oxysterol receptor LXRβ protects against DSS- and TNBS-induced colitis in mice. Mucosal Immunology 7, 1416–1428 (2014).
- Wagner, A. E. et al. DSS-induced acute colitis in C57BL/6 mice is mitigated by sulforaphane pre-treatment. The Journal of Nutritional Biochemistry 24, 2085–2091 (2013).
- Thaker, A. I. et al. IDO1 Metabolites Activate β-catenin Signaling to Promote Cancer Cell Proliferation and Colon Tumorigenesis in Mice. Gastroenterology 145, 416-425.e4 (2013).
- Schreiber, O. et al. iNOS-Dependent Increase in Colonic Mucus Thickness in DSS-Colitic Rats. PLOS ONE 8, e71843 (2013).
- Ranganathan, P., Jayakumar, C., Santhakumar, M. & Ramesh, G. Netrin-1 regulates colon-kidney cross talk through suppression of IL-6 function in a mouse model of DSS-colitis. American Journal of Physiology-Renal Physiology 304, F1187–F1197 (2013).
- Konieczna, P. et al. Immunomodulation by Bifidobacterium infantis 35624 in the Murine Lamina Propria Requires Retinoic Acid-Dependent and Independent Mechanisms. PLOS ONE 8, e62617 (2013).
- Konieczna, P. et al. Immunomodulation by Bifidobacterium infantis 35624 in the Murine Lamina Propria Requires Retinoic Acid-Dependent and Independent Mechanisms. PLOS ONE 8, e62617 (2013).
- Hall, L. J. et al. Natural killer cells protect mice from DSS-induced colitis by regulating neutrophil function via the NKG2A receptor. Mucosal Immunology 6, 1016–1026 (2013).
- Guzman, J. R. et al. Oxymatrine Prevents NF-κB Nuclear Translocation And Ameliorates Acute Intestinal Inflammation. Scientific Reports 3, 1629 (2013).
- Brounais-Le Royer, B. et al. Effects of an Interleukin-15 Antagonist on Systemic and Skeletal Alterations in Mice with DSS-Induced Colitis. The American Journal of Pathology 182, 2155–2167 (2013).
- Beloqui, A. et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. International Journal of Pharmaceutics 454, 775–783 (2013).
- Ackermann, M., Tsuda, A., Secomb, T. W., Mentzer, S. J. & Konerding, M. A. Intussusceptive remodeling of vascular branch angles in chemically-induced murine colitis. Microvascular Research 87, 75–82 (2013).
- Øines, E., Murison, R., Mrdalj, J., Grønli, J. & Milde, A. M. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiology & Behavior 105, 1058–1066 (2012).
- Xu, C., Shen, Y., Littman, D. R., Dustin, M. L. & Velázquez, P. Visualization of mucosal homeostasis via single- and multiphoton intravital fluorescence microscopy. Journal of Leukocyte Biology 92, 413–419 (2012).
- Wadie, W. et al. STW 5 is effective in dextran sulfate sodium-induced colitis in rats. Int J Colorectal Dis 27, 1445–1453 (2012).
- Thaker, A. I., Shaker, A., Rao, M. S. & Ciorba, M. A. Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS). J Vis Exp (2012). doi:10.3791/4100
- Thaker, A. I., Shaker, A., Rao, M. S. & Ciorba, M. A. Modeling Colitis-Associated Cancer with Azoxymethane (AOM) and Dextran Sulfate Sodium (DSS). JoVE (Journal of Visualized Experiments) e4100 (2012). doi:10.3791/4100
- Takiguchi, H. et al. Reduced production of polymeric immunoglobulin receptor in murine dextran sodium sulfate-induced colitis. Journal of Oral Science 54, 23–32 (2012).
- Spadoni, I., Iliev, I. D., Rossi, G. & Rescigno, M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunology 5, 184–193 (2012).
- Rang, S. et al. Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction. ERA (2012). doi:10.7939/R3DJ58W4W
- Philipp, E. E. R. et al. Massively Parallel RNA Sequencing Identifies a Complex Immune Gene Repertoire in the lophotrochozoan Mytilus edulis. PLOS ONE 7, e33091 (2012).
- Lied, G. A. et al. Increased wall thickness using ultrasonography is associated with inflammation in an animal model of experimental colitis. Clin Exp Gastroenterol 5, 195–201 (2012).
- Heinsbroek, S. E. et al. Genetic deletion of dectin-1 does not affect the course of murine experimental colitis. BMC Gastroenterol 12, 33 (2012).
- Grimstad, T. et al. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scandinavian Journal of Gastroenterology 47, 49–58 (2012).
- Gottfries, J., Melgar, S. & Michaëlsson, E. Modelling of Mouse Experimental Colitis by Global Property Screens: A Holistic Approach to Assess Drug Effects in Inflammatory Bowel Disease. PLOS ONE 7, e30005 (2012).
- Ding, S. et al. Mucosal Healing and Fibrosis after Acute or Chronic Inflammation in Wild Type FVB-N Mice and C57BL6 Procollagen α1(I)-Promoter-GFP Reporter Mice. PLOS ONE 7, e42568 (2012).
- Dicksved, J. et al. Lactobacillus reuteri Maintains a Functional Mucosal Barrier during DSS Treatment Despite Mucus Layer Dysfunction. PLOS ONE 7, e46399 (2012).
- DeBoer, M. D., Steinman, J. & Li, Y. Partial normalization of pubertal timing in female mice with DSS colitis treated with anti-TNF-α antibody. J Gastroenterol 47, 647–654 (2012).
- de Jong, J. H. et al. Fusion of intestinal epithelial cells with bone marrow derived cells is dispensable for tissue homeostasis. Scientific Reports 2, 271 (2012).
- Borniquel, S., Jädert, C. & Lundberg, J. O. Dietary Conjugated Linoleic Acid Activates PPARγ and the Intestinal Trefoil Factor in SW480 Cells and Mice with Dextran Sulfate Sodium-Induced Colitis. J Nutr 142, 2135–2140 (2012).
2011-2000
- Villegas, I., Sánchez‐Fidalgo, S. & Lastra, C. A. de la. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Molecular Nutrition & Food Research 55, 259–267 (2011).
- Rahman, A. et al. Chronic colitis induces expression of β-defensins in murine intestinal epithelial cells. Clinical & Experimental Immunology 163, 123–130 (2011).
- Queiroz, K. C. S. et al. Tissue Factor-Dependent Chemokine Production Aggravates Experimental Colitis. Mol Med 17, 1119–1126 (2011).
- Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. PNAS 108, 9601–9606 (2011).
- Lenoir, L. et al. Lemon Verbena Infusion Consumption Attenuates Oxidative Stress in Dextran Sulfate Sodium-Induced Colitis in the Rat. Dig Dis Sci 56, 3534–3545 (2011).
- Hall, L. J. et al. Induction and Activation of Adaptive Immune Populations During Acute and Chronic Phases of a Murine Model of Experimental Colitis. Dig Dis Sci 56, 79–89 (2011).
- Deboer, M. D. & Li, Y. Puberty Is Delayed in Male Mice With Dextran Sodium Sulfate Colitis Out of Proportion to Changes in Food Intake, Body Weight, and Serum Levels of Leptin. Pediatric Research 69, 34–39 (2011).
- Cançado, G. G. L. et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. Inflamm Bowel Dis 17, 2275–2286 (2011).
- Banerjee, S. et al. Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. American Journal of Physiology-Gastrointestinal and Liver Physiology 300, G273–G282 (2011).
- van Dop, W. A. et al. The absence of functional PI3Kγ prevents leukocyte recruitment and ameliorates DSS-induced colitis in mice. Immunology Letters 131, 33–39 (2010).
- Snoek, S. A. et al. Selective α7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. British Journal of Pharmacology 160, 322–333 (2010).
- Sina, C. et al. Ablation of gly96/immediate early gene-X1 (gly96/iex-1) aggravates DSS-induced colitis in mice: Role for gly96/iex-1 in the regulation of NF-κB. Inflamm Bowel Dis 16, 320–331 (2010).
- Sánchez-Fidalgo, S. et al. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clinical Nutrition 29, 663–673 (2010).
- Petersson, J. et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 300, G327–G333 (2010).
- Murphy, C. T. et al. Technical Advance: Function and efficacy of an α4-integrin antagonist using bioluminescence imaging to detect leukocyte trafficking in murine experimental colitis. Journal of Leukocyte Biology 88, 1271–1278 (2010).
- Murphy, C. T. et al. Technical Advance: Function and efficacy of an α4-integrin antagonist using bioluminescence imaging to detect leukocyte trafficking in murine experimental colitis. Journal of Leukocyte Biology 88, 1271–1278 (2010).
- Murphy, C. T. et al. Use of bioluminescence imaging to track neutrophil migration and its inhibition in experimental colitis. Clinical & Experimental Immunology 162, 188–196 (2010).
- Konerding, M. A. et al. Inflammation-Induced Intussusceptive Angiogenesis in Murine Colitis. The Anatomical Record 293, 849–857 (2010).
- Johansson, M. E. V. et al. Bacteria Penetrate the Inner Mucus Layer before Inflammation in the Dextran Sulfate Colitis Model. PLOS ONE 5, e12238 (2010).
- Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. Journal of Experimental Medicine 207, 823–836 (2010).
- DeBoer, M. D., Li, Y. & Cohn, S. Colitis causes delay in puberty in female mice out of proportion to changes in leptin and corticosterone. J Gastroenterol 45, 277–284 (2010).
- Ciorba, M. A. et al. Induction of IDO-1 by Immunostimulatory DNA Limits Severity of Experimental Colitis. The Journal of Immunology 184, 3907–3916 (2010).
- Cadwell, K. et al. Virus-Plus-Susceptibility Gene Interaction Determines Crohn’s Disease Gene Atg16L1 Phenotypes in Intestine. Cell 141, 1135–1145 (2010).
- Borniquel, S., Jansson, E. Å., Cole, M. P., Freeman, B. A. & Lundberg, J. O. Nitrated oleic acid up-regulates PPARγ and attenuates experimental inflammatory bowel disease. Free Radical Biology and Medicine 48, 499–505 (2010).
- Zheng, L., Riehl, T. E. & Stenson, W. F. Regulation of Colonic Epithelial Repair in Mice by Toll-Like Receptors and Hyaluronic Acid. Gastroenterology 137, 2041–2051 (2009).
- Turhan, A. et al. Vascular Microarchitecture of Murine Colitis-Associated Lymphoid Angiogenesis. The Anatomical Record 292, 621–632 (2009).
- Tsuda, A. et al. Bimodal Oscillation Frequencies of Blood Flow in the Inflammatory Colon Microcirculation. The Anatomical Record 292, 65–72 (2009).
- Sina, C. et al. G Protein-Coupled Receptor 43 Is Essential for Neutrophil Recruitment during Intestinal Inflammation. The Journal of Immunology 183, 7514–7522 (2009).
- Schreiber, O. et al. Lactobacillus reuteri prevents colitis by reducing P-selectin-associated leukocyte- and platelet-endothelial cell interactions. American Journal of Physiology-Gastrointestinal and Liver Physiology 296, G534–G542 (2009).
- Miele, L. F. et al. Blood Flow Patterns Spatially Associated with Platelet Aggregates in Murine Colitis. The Anatomical Record 292, 1143–1153 (2009).
- Lång, P., Lange, S., Delbro, D. & Andersson, G. Induction and cellular expression of tartrate resistant acid phosphatase during dextran sodium sulphate induced colitis in rats. Histochem Cell Biol 132, 599 (2009).
- Iliev, I. D., Mileti, E., Matteoli, G., Chieppa, M. & Rescigno, M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunology 2, 340–350 (2009).
- Huang, T.-Y. et al. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicology and Applied Pharmacology 237, 69–82 (2009).
- Hansen, J. J., Holt, L. & Sartor, B. R. Gene Expression Patterns in Experimental Colitis in IL-10-Deficient Mice. Inflamm Bowel Dis 15, 890–899 (2009).
- Davies, P. S., Powell, A. E., Swain, J. R. & Wong, M. H. Inflammation and Proliferation Act Together to Mediate Intestinal Cell Fusion. PLOS ONE 4, e6530 (2009).
- Canevari, M. et al. Poly(ethylene glycol)-mesalazine conjugate for colon specific delivery. International Journal of Pharmaceutics 368, 171–177 (2009).
- Banerjee, S. et al. MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease. Mucosal Immunol 2, 220–231 (2009).
- Voltan, S. et al. Lactobacillus crispatus M247-Derived H2O2 Acts as a Signal Transducing Molecule Activating Peroxisome Proliferator Activated Receptor-γ in the Intestinal Mucosa. Gastroenterology 135, 1216–1227 (2008).
- Turhan, A. et al. Bridging Mucosal Vessels Associated with Rhythmically Oscillating Blood Flow in Murine Colitis. The Anatomical Record 291, 74–82 (2008).
- Ramakers, J. D., Mensink, R. P., Verstege, M. I., Velde, A. A. te & Plat, J. An arachidonic acid-enriched diet does not result in more colonic inflammation as compared with fish oil- or oleic acid-enriched diets in mice with experimental colitis. British Journal of Nutrition 100, 347–354 (2008).
- Melgar, S. et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. International Immunopharmacology 8, 836–844 (2008).
- Melgar, S., Engström, K., Jägervall, Å. & Martinez, V. Psychological stress reactivates dextran sulfate sodium-induced chronic colitis in mice. Stress 11, 348–362 (2008).
- Matters, G. L. et al. The Opioid Antagonist Naltrexone Improves Murine Inflammatory Bowel Disease. Journal of Immunotoxicology 5, 179–187 (2008).
- Li, H. et al. Intestinal, adipose, and liver inflammation in diet-induced obese mice. Metabolism 57, 1704–1710 (2008).
- Karlsson, A. et al. Dextran sulphate sodium induces acute colitis and alters hepatic function in hamsters. International Immunopharmacology 8, 20–27 (2008).
- Fritsch Fredin, M. et al. The application and relevance of ex vivo culture systems for assessment of IBD treatment in murine models of colitis. Pharmacological Research 58, 222–231 (2008).
- Spek, A. C., Kate, F. J. W. ten & Velde, A. A. te. Factor V Leiden and the etiology of inflammatory bowel disease. Thromb Haemost 98, 670–673 (2007).
- Rigby, R. J., Simmons, J. G., Greenhalgh, C. J., Alexander, W. S. & Lund, P. K. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene 26, 4833–4841 (2007).
- Ramakers, J. D. et al. The PPARγ Agonist Rosiglitazone Impairs Colonic Inflammation in Mice with Experimental Colitis. J Clin Immunol 27, 275–283 (2007).
- Petersson, J. et al. eNOS involved in colitis-induced mucosal blood flow increase. American Journal of Physiology-Gastrointestinal and Liver Physiology 293, G1281–G1287 (2007).
- Nysœter, G. et al. Effect of live Salmonella Ty21a in Dextran Sulfate Sodium-induced Colitis. Drug Target�Insights 2, DTI.S220 (2007).
- Melgar, S., Gillberg, P.-G., Hockings, P. D. & Olsson, L. E. High-throughput magnetic resonance imaging in murine colonic inflammation. Biochemical and Biophysical Research Communications 355, 1102–1107 (2007).
- Melgar, S. et al. Mice with experimental colitis show an altered metabolism with decreased metabolic rate. American Journal of Physiology-Gastrointestinal and Liver Physiology 292, G165–G172 (2007).
- Holma, R., Salmenperä, P., Virtanen, I., Vapaatalo, H. & Korpela, R. Prophylactic potential of montelukast against mild colitis induced by dextran sulphate sodium in rats. J. Physiol. Pharmacol. 58, 455–467 (2007).
- Brown, S. L. et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 117, 258–269 (2007).
- Arslan, G. et al. No Protection against DSS-induced Colitis by Short-term Pretreatment with Seal or Fish Oils in Rats. Integr Med�Insights 2, 117863370700200000 (2007).
- Van der Sluis, M. et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 131, 117–129 (2006).
- Lundberg, S., Lindholm, J., Lindbom, L., Hellström, P. M. & Werr, J. Integrin α2β1 Regulates Neutrophil Recruitment and Inflammatory Activity in Experimental Colitis in Mice. Inflamm Bowel Dis 12, 172–177 (2006).
- Larsson, M. H., Rapp, L. & Lindström, E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterology & Motility 18, 144–152 (2006).
- Larsson, A. E. et al. Magnetic resonance imaging of experimental mouse colitis and association with inflammatory activity. Inflamm Bowel Dis 12, 478–485 (2006).
- Giannakis, M. et al. Molecular Properties of Adult Mouse Gastric and Intestinal Epithelial Progenitors in Their Niches. J. Biol. Chem. 281, 11292–11300 (2006).
- Chidlow, J. H. et al. Differential Angiogenic Regulation of Experimental Colitis. The American Journal of Pathology 169, 2014–2030 (2006).
- Brand, S. et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. American Journal of Physiology-Gastrointestinal and Liver Physiology 290, G827–G838 (2006).
- Sund, M. et al. Reduced susceptibility to dextran sulphate sodium-induced colitis in the interleukin-2 heterozygous (IL-2+/–) mouse. Immunology 114, 554–564 (2005).
- Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. & Stappenbeck, T. S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. PNAS 102, 99–104 (2005).
- Milde, A. M., Arslan, G., Overmier, J. B., Berstad, A. & Murison, R. An acute stressor enhances sensitivity to a chemical irritant and increases51CrEDTA permeability of the colon in adult rats. Integr. psych. behav. 40, 35–44 (2005).
- Elrod, J. W. et al. DSS-Induced Colitis Is Exacerbated in STAT-6 Knockout Mice. Inflamm Bowel Dis 11, 883–889 (2005).
- Castagliuolo, I. et al. Beneficial effect of auto-aggregating Lactobacillus crispatus on experimentally induced colitis in mice. FEMS Immunol Med Microbiol 43, 197–204 (2005).
- Brun, P. et al. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology 288, G621–G629 (2005).
- Bennink, R. J. et al. Dedicated Pinhole SPECT of Intestinal Neutrophil Recruitment in a Mouse Model of Dextran Sulfate Sodium–Induced Colitis. J Nucl Med 46, 526–531 (2005).
- Leemans, J. C. et al. The Epidermal Growth Factor-Seven Transmembrane (EGF-TM7) Receptor CD97 Is Required for Neutrophil Migration and Host Defense. The Journal of Immunology 172, 1125–1131 (2004).
- Edalat, M., Mannervik, B. & Axelsson, L.-G. Selective expression of detoxifying glutathione transferases in mouse colon: effect of experimental colitis and the presence of bacteria. Histochem Cell Biol 122, 151–159 (2004).
- Bene, L. et al. Partial Protection against Dextran Sodium Sulphate Induced Colitis in Histamine-Deficient, Histidine Decarboxylase Knockout Mice. Journal of Pediatric Gastroenterology and Nutrition 39, 171 (2004).
- Sasaki, M. et al. The 3-Hydroxy-3-methylglutaryl-CoA Reductase Inhibitor Pravastatin Reduces Disease Activity and Inflammation in Dextran-Sulfate Induced Colitis. J Pharmacol Exp Ther 305, 78–85 (2003).
- Sasaki, M. et al. The 3-Hydroxy-3-methylglutaryl-CoA Reductase Inhibitor Pravastatin Reduces Disease Activity and Inflammation in Dextran-Sulfate Induced Colitis. J Pharmacol Exp Ther 305, 78–85 (2003).
- Sasaki, M. et al. Increased disease activity in eNOS-deficient mice in experimental colitis. Free Radical Biology and Medicine 35, 1679–1687 (2003).
- Milde, A. M., Sundberg, H., RØseth, A. G. & Murison, R. Proactive Sensitizing Effects of Acute Stress on Acoustic Startle Responses and Experimentally Induced Colitis in Rats: Relationship to Corticosterone. Stress 6, 49–57 (2003).
- Milde, A. M., Sundberg, H., RØseth, A. G. & Murison, R. Proactive Sensitizing Effects of Acute Stress on Acoustic Startle Responses and Experimentally Induced Colitis in Rats: Relationship to Corticosterone. Stress 6, 49–57 (2003).
- Börjesson, L. & Delbro, D. S. Neurogenic and non-neurogenic mechanisms in response of rat distal colon muscle to dextran sulphate sodium treatment. Autonomic Neuroscience 107, 74–80 (2003).
- Spiik, A.-K. et al. Abrogated lymphocyte infiltration and lowered CD14 in dextran sulfate induced colitis in mice treated with p65 antisense oligonucleotides. Int J Colorectal Dis 17, 223–232 (2002).
- Milde, A. M. & Murison, R. A study of the effets of restraint stress on colitis induced by dextran sulphate sodium in singly housed rats. Integrative Physiological & Behavioral Science 37, 140–150 (2002).
- Castagliuolo, I. et al. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. British Journal of Pharmacology 136, 271–279 (2002).
- Williams, K. L. et al. Enhanced survival and mucosal repair after dextran sodium sulfate–induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology 120, 925–937 (2001).
- Stevceva, L., Pavli, P., Husband, A., Ramsay, A. & Doe, W. F. Dextran sulphate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Genes Immun 2, 309–316 (2001).
- Börjesson, L., Aldenborg, F. & Delbro, D. S. Functional effects of dextran sulphate sodium (DSS) treatment on the longitudinal muscle of rat distal colon. Journal of Autonomic Pharmacology 21, 121–129 (2001).
- Stevceva, L. et al. Eosinophilia is attenuated in experimental colitis induced in IL-5 deficient mice. Genes Immun 1, 213–218 (2000).
- Morteau, O. et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest 105, 469–478 (2000).
1999-1994
- Stevceva, L., Pavli, P., Buffinton, G., Wozniak, A. & Doe, W. Dextran sodium sulphate-induced colitis activity varies with mouse strain but develops in lipopolysaccharide-unresponsive mice. Journal of Gastroenterology and Hepatology 14, 54–60 (1999).
- Mähler, M. et al. Genetic Analysis of Susceptibility to Dextran Sulfate Sodium-Induced Colitis in Mice. Genomics 55, 147–156 (1999).
- Blackburn, A. C., Doe, W. F. & Buffinton, G. D. Protein carbonyl formation on mucosal proteins in vitro and in dextran sulfate-induced colitis. Free Radical Biology and Medicine 27, 262–270 (1999).
- Mähler, M. et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 274, G544–G551 (1998).
- Dieleman, L. A. et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114, 385–391 (1998).
- Blackburn, A. C., Doe, W. F. & Buffinton, G. D. Salicylate Hydroxylation as an Indicator of Hydroxyl Radical Generation in Dextran Sulfate-Induced Colitis. Free Radical Biology and Medicine 25, 305–313 (1998).
- Axelsson, L.-G., Landström, E. & Bylund‐Fellenius, A.-C. Experimental colitis induced by dextran sulphate sodium in mice: beneficial effects of sulphasalazine and olsalazine. Alimentary Pharmacology & Therapeutics 12, 925–934 (1998).
- Blackburn, A. C., Doe, W. F. & Buffinton, G. D. Colonic antioxidant status in dextran sulfate-induced colitis in mice. Inflammatory Bowel Diseases 3, 198–203 (1997).
- Lange, S., Delbro, D. S., Jennische, E. & Mattsby‐Baltzer, I. The role of the Lps gene in experimental ulcerative colitis in mice. APMIS 104, 823–833 (1996).
- Axelsson, L.-G., Midtvedt, T. & Bylund-Fellenius, A.-C. The Role of Intestinal Bacteria, Bacterial Translocation and Endotoxin in Dextran Sodium Sulphate-Induced Colitis in the Mouse. Microbial Ecology in Health and Disease 9, 225–237 (1996).
- Axelsson, L.-G., Landström, E., Goldschmidt, T. J., Grönberg, A. & Bylund-Fellenius, A.-C. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: Effects in CD4+-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res 45, 181–191 (1996).
- Dieleman, L. A. et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107, 1643–1652 (1994).
- Willemze, R. A. et al. Acetylcholine-producing T-cells augment innate immune driven colitis but are redundant in T-cell driven colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology (2019) doi:10.1152/ajpgi.00067.2019.
- Wéra, O. et al. P2X1 ion channel deficiency causes massive bleeding in inflamed intestine and increases thrombosis. Journal of Thrombosis and Haemostasis 0, (2019).
- Wang, Y. et al. Long-Term Culture Captures Injury-Repair Cycles of Colonic Stem Cells. Cell 179, 1144-1159.e15 (2019).
- Wang, Y. et al. Long-Term Culture Captures Injury-Repair Cycles of Colonic Stem Cells. Cell (2019) doi:10.1016/j.cell.2019.10.015.
- Volk, J. K. et al. The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function. Journal of Experimental Medicine jem.20190679 (2019) doi:10.1084/jem.20190679.
- Volarevic, V. et al. Galectin-3 Regulates Indoleamine-2,3-dioxygenase-Dependent Cross-Talk between Colon-Infiltrating Dendritic Cells and T Regulatory Cells and May Represent a Valuable Biomarker for Monitoring the Progression of Ulcerative Colitis. Cells 8, 709 (2019).
- Staats, S. et al. Dietary ursolic acid improves health span and life span in male Drosophila melanogaster. BioFactors 45, 169–186 (2019).
- Singh, K. et al. Dietary Arginine Regulates Severity of Experimental Colitis and Affects the Colonic Microbiome. Front. Cell. Infect. Microbiol. 9, (2019).
- Samba-Mondonga, M., Constante, M., Fragoso, G., Calvé, A. & Santos, M. M. Curcumin induces mild anemia in a DSS-induced colitis mouse model maintained on an iron-sufficient diet. PLOS ONE 14, e0208677 (2019).
- Salmenkari, H. et al. The use of unlicensed bone marrow–derived platelet lysate–expanded mesenchymal stromal cells in colitis: a pre-clinical study. Cytotherapy 21, 175–188 (2019).
- Polari, L. et al. Novel Selective Estrogen Receptor Modulator Ameliorates Murine Colitis. International Journal of Molecular Sciences 20, 3007 (2019).
- Neil, J. A. et al. IFN-I and IL-22 mediate protective effects of intestinal viral infection. Nature Microbiology 1 (2019) doi:10.1038/s41564-019-0470-1.
- Markovic, M. et al. Phospholipid-Based Prodrugs for Colon-Targeted Drug Delivery: Experimental Study and In-Silico Simulations. Pharmaceutics 11, 186 (2019).
- Lleal, M. et al. A single faecal microbiota transplantation modulates the microbiome and improves clinical manifestations in a rat model of colitis. EBioMedicine (2019) doi:10.1016/j.ebiom.2019.10.002.
- Leleu-Chavain, N. et al. Benzo[d]thiazol-2(3H)-ones as new potent selective CB2 agonists with anti-inflammatory properties. European Journal of Medicinal Chemistry 165, 347–362 (2019).
- Jofra, T. et al. Experimental colitis in IL-10-deficient mice ameliorates in the absence of PTPN22. Clin. Exp. Immunol. 197, 263–275 (2019).
- Guo, Y. et al. Loss of cyclin A2 in murine colonic epithelial cells disrupts colon homeostasis by triggering DNA damage and dysplasia and high cyclin A2 expression is a good-prognosis factor in patients with colorectal cancer. bioRxiv 690404 (2019) doi:10.1101/690404.
- Guillemot-Legris, O. et al. Colitis Alters Oxysterol Metabolism and is Affected by 4β-Hydroxycholesterol Administration. J Crohns Colitis 13, 218–229 (2019).
- Gowrikumar, S. et al. Upregulated claudin-1 expression promotes colitis-associated cancer by promoting β-catenin phosphorylation and activation in Notch/p-AKT-dependent manner. Oncogene 38, 5321 (2019).
- Garibay, D. et al. TGR5 Protects Against Colitis in Mice, but Vertical Sleeve Gastrectomy Increases Colitis Severity. OBES SURG 29, 1593–1601 (2019).
- Friedrich, M. et al. HDAC inhibitors promote intestinal epithelial regeneration via autocrine TGFβ1 signalling in inflammation. Mucosal Immunology 12, 656 (2019).
- Eshelman, M. A. et al. Tristetraprolin targets Nos2 expression in the colonic epithelium. Sci Rep 9, 1–13 (2019).
- Durmus, S. et al. ABC transporters Mdr1a/1b, Bcrp1, Mrp2 and Mrp3 determine the sensitivity to PhIP/DSS-induced colon carcinogenesis and inflammation. Arch Toxicol 93, 775–790 (2019).
- Dempsey, E., Abautret-Daly, Á., Docherty, N. G., Medina, C. & Harkin, A. Persistent central inflammation and region specific cellular activation accompany depression- and anxiety-like behaviours during the resolution phase of experimental colitis. Brain, Behavior, and Immunity (2019) doi:10.1016/j.bbi.2019.05.007.
- De Vries, L. C. S. et al. A JAK1 Selective Kinase Inhibitor and Tofacitinib Affect Macrophage Activation and Function. Inflamm Bowel Dis 25, 647–660 (2019).
- Cribiù, F. M. et al. Using Robotic Systems to Process and Embed Colonic Murine Samples for Histological Analyses. JoVE (Journal of Visualized Experiments) e58654 (2019) doi:10.3791/58654.
- Coburn, L. A. et al. Loss of solute carrier family 7 member 2 exacerbates inflammation-associated colon tumorigenesis. Oncogene 38, 1067 (2019).
- Chen, Y., Zhang, M. & Ren, F. A Role of Exopolysaccharide Produced by Streptococcus thermophilus in the Intestinal Inflammation and Mucosal Barrier in Caco-2 Monolayer and Dextran Sulphate Sodium-Induced Experimental Murine Colitis. Molecules 24, 513 (2019).
- Chang, Y.-L. et al. Therapeutic Efficacy of Subcutaneous and Intraperitoneal Injections of a Single Dose of Human Umbilical Mesenchymal Stem Cells in Acute and Chronic Colitis in a Mouse Model. J. Med. Biol. Eng. (2019) doi:10.1007/s40846-019-00494-7.
- Chang, Y.-L. et al. Therapeutic effects of a single injection of human umbilical mesenchymal stem cells on acute and chronic colitis in mice. Scientific Reports 9, 5832 (2019).
- Chang, C.-L. et al. Synergistic effect of combined melatonin and adipose-derived mesenchymal stem cell (ADMSC)-derived exosomes on amelioration of dextran sulfate sodium (DSS)-induced acute colitis. Am J Transl Res 11, 2706–2724 (2019).
- Casado‐Bedmar, M., Heil, S. D. S., Myrelid, P., Söderholm, J. D. & Keita, Å. V. Upregulation of intestinal mucosal mast cells expressing VPAC1 in close proximity to vasoactive intestinal polypeptide in inflammatory bowel disease and murine colitis. Neurogastroenterology & Motility 31, e13503 (2019).
- Burrello, C. et al. Mucosa-associated microbiota drives pathogenic functions in IBD-derived intestinal iNKT cells. Life Science Alliance 2, e201800229 (2019).
- Burrello, C. et al. Fecal Microbiota Transplantation Controls Murine Chronic Intestinal Inflammation by Modulating Immune Cell Functions and Gut Microbiota Composition. Cells 8, 517 (2019).
- Body-Malapel, M. et al. The RAGE signaling pathway is involved in intestinal inflammation and represents a promising therapeutic target for Inflammatory Bowel Diseases. Mucosal Immunology 12, 468 (2019).
- Al-Omari, M. M., Razan B. Al-Ghariebeh, A. A. A. A., Zoubi, H. A.- & Al-Qaoud, K. M. Camel milk Whey Inhibits Inflammatory Colorectal Cancer Development Via Down regulation of Pro-inflammatory Cytokines in Induced AOM/DSS Mouse Model. 1 256–262 (2019) doi:10.9755/ejfa.2019.v31.i4.1935.
- Aden, K. et al. Epithelial RNase H2 Maintains Genome Integrity and Prevents Intestinal Tumorigenesis in Mice. Gastroenterology 156, 145-159.e19 (2019).
- Willemze, R. A. et al. Neuronal control of experimental colitis occurs via sympathetic intestinal innervation. Neurogastroenterology & Motility 30, e13163 (2018).
- Singh, A. K., Hertzberger, R. Y. & Knaus, U. G. Hydrogen peroxide production by lactobacilli promotes epithelial restitution during colitis. Redox Biology 16, 11–20 (2018).
- Sferra, R. et al. Interaction between sphingosine kinase/sphingosine 1 phosphate and transforming growth factor-β/Smads pathways in experimental intestinal fibrosis. An in vivo immunohistochemical study. Eur J Histochem 62, (2018).
- Salmenkari, H., Pasanen, L., Linden, J., Korpela, R. & Vapaatalo, H. Beneficial anti-inflammatory effect of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in the treatment of dextran sulfate sodium-induced colitis in mice. J. Physiol. Pharmacol. 69, (2018).
- Radulovic, K. et al. A dietary flavone confers communicable protection against colitis through NLRP6 signaling independently of inflammasome activation. Mucosal Immunol 11, 811–819 (2018).
- Paveljšek, D. et al. Lactobacillus fermentum L930BB and Bifidobacterium animalis subsp. animalis IM386 initiate signalling pathways involved in intestinal epithelial barrier protection. Beneficial Microbes 9, 515–525 (2018).
- Nikolic, A. et al. Intraperitoneal administration of mesenchymal stem cells ameliorates acute dextran sulfate sodium-induced colitis by suppressing dendritic cells. Biomedicine & Pharmacotherapy 100, 426–432 (2018).
- Messal, N. et al. Ectopic expression of OX1R in ulcerative colitis mediates anti-inflammatory effect of orexin-A. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 1864, 3618–3628 (2018).
- Masquelier, J. et al. Lysophosphatidylinositols in inflammation and macrophage activation: Altered levels and anti-inflammatory effects. Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids 1863, 1458–1468 (2018).
- Liu, X. et al. 1-L-MT, an IDO inhibitor, prevented colitis-associated cancer by inducing CDC20 inhibition-mediated mitotic death of colon cancer cells. International Journal of Cancer 143, 1516–1529 (2018).
- Koren, E. et al. ARTS mediates apoptosis and regeneration of the intestinal stem cell niche. Nature Communications 9, 4582 (2018).
- Kesharwani, S. S. et al. Site-directed non-covalent polymer-drug complexes for inflammatory bowel disease (IBD): Formulation development, characterization and pharmacological evaluation. Journal of Controlled Release 290, 165–179 (2018).
- K. Martin, P. et al. Autophagy proteins suppress protective type I interferon signaling in response to the murine gut microbiota. Nature Microbiology 3, (2018).
- Greicius, G. et al. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. PNAS 115, E3173–E3181 (2018).
- Gobert, A. P. et al. Distinct Immunomodulatory Effects of Spermine Oxidase in Colitis Induced by Epithelial Injury or Infection. Front. Immunol. 9, (2018).
- Gaifem, J. et al. L-Threonine Supplementation During Colitis Onset Delays Disease Recovery. Front. Physiol. 9, (2018).
- Farombi, E. O. et al. 6-Gingerol improves testicular function in mice model of chronic ulcerative colitis. Hum Exp Toxicol 37, 358–372 (2018).
- Fan, T.-J. et al. Environmental Factors Modify the Severity of Acute DSS Colitis in Caspase-11-Deficient Mice. Inflamm Bowel Dis 24, 2394–2403 (2018).
- Ducheix, S. et al. Deletion of Stearoyl-CoA Desaturase-1 From the Intestinal Epithelium Promotes Inflammation and Tumorigenesis, Reversed by Dietary Oleate. Gastroenterology 155, 1524-1538.e9 (2018).
- Darnaud, M. et al. Enteric Delivery of Regenerating Family Member 3 alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice With Colitis. Gastroenterology 154, 1009-1023.e14 (2018).
- Da Silva, S. et al. A Novel Topical PPARγ Agonist Induces PPARγ Activity in Ulcerative Colitis Mucosa and Prevents and Reverses Inflammation in Induced Colitis Models. Inflamm Bowel Dis 24, 792–805 (2018).
- Cuellar-Nuñez, M. L. et al. Physicochemical and nutraceutical properties of moringa (Moringa oleifera) leaves and their effects in an in vivo AOM/DSS-induced colorectal carcinogenesis model. Food Research International 105, 159–168 (2018).
- Cribiù, F. M. et al. Implementation of an automated inclusion system for the histological analysis of murine tissue samples: A feasibility study in DSS-induced chronic colitis. Eur J Inflamm 16, 2058739218776883 (2018).
- Cardoso, A. et al. The Dynamics of Interleukin-10-Afforded Protection during Dextran Sulfate Sodium-Induced Colitis. Front. Immunol. 9, (2018).
- Burrello, C. et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nature Communications 9, 5184 (2018).
- Burrello, C. et al. Short-term Oral Antibiotics Treatment Promotes Inflammatory Activation of Colonic Invariant Natural Killer T and Conventional CD4+ T Cells. Front. Med. 5, (2018).
- Alhouayek, M., Buisseret, B., Paquot, A., Guillemot-Legris, O. & Muccioli, G. G. The endogenous bioactive lipid prostaglandin D2-glycerol ester reduces murine colitis via DP1 and PPARγ receptors. The FASEB Journal 32, 5000–5011 (2018).
- Ajayi, B., Adedara, I. & Farombi, E. Protective mechanisms of 6-gingerol in dextran sulfate sodium-induced chronic ulcerative colitis in mice. Hum Exp Toxicol 37, 1054–1068 (2018).
- Ajayi, B. O., Adedara, I. A., Ajani, O. S., Oyeyemi, M. O. & Farombi, E. O. [6]-Gingerol modulates spermatotoxicity associated with ulcerative colitis and benzo[a]pyrene exposure in BALB/c mice. Journal of Basic and Clinical Physiology and Pharmacology 29, 247–256 (2018).
- Acovic, A. et al. Indoleamine 2,3-dioxygenase-dependent expansion of T-regulatory cells maintains mucosal healing in ulcerative colitis. Therap Adv Gastroenterol 11, 1756284818793558 (2018).
- Zhdanov, A. V. et al. Quantitative analysis of mucosal oxygenation using ex vivo imaging of healthy and inflamed mammalian colon tissue. Cell. Mol. Life Sci. 74, 141–151 (2017).
- Udden, S. M. N. et al. NOD2 Suppresses Colorectal Tumorigenesis via Downregulation of the TLR Pathways. Cell Reports 19, 2756–2770 (2017).
- Tubbs, A. L., Liu, B., Rogers, T. D., Sartor, R. B. & Miao, E. A. Dietary Salt Exacerbates Experimental Colitis. The Journal of Immunology 199, 1051–1059 (2017).
- Sünderhauf, A. et al. Regulation of epithelial cell expressed C3 in the intestine – Relevance for the pathophysiology of inflammatory bowel disease? Molecular Immunology 90, 227–238 (2017).
- Štofilová, J. et al. Cytokine production in vitro and in rat model of colitis in response to Lactobacillus plantarum LS/07. Biomedicine & Pharmacotherapy 94, 1176–1185 (2017).
- Smole, A., Lainšček, D., Bezeljak, U., Horvat, S. & Jerala, R. A Synthetic Mammalian Therapeutic Gene Circuit for Sensing and Suppressing Inflammation. Molecular Therapy 25, 102–119 (2017).
- Pagel, R. et al. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. The FASEB Journal 31, 4707–4719 (2017).
- O’Sullivan, S. et al. Inhibition of matrix metalloproteinase-9 by a barbiturate-nitrate hybrid ameliorates dextran sulphate sodium-induced colitis: effect on inflammation-related genes: Nitrate inhibition of MMP-9 in DSS-induced colitis. British Journal of Pharmacology 174, 512–524 (2017).
- Menghini, P. et al. A novel model of colitis-associated cancer in SAMP1/YitFc mice with Crohn’s disease-like ileitis. PLoS ONE 12, e0174121 (2017).
- Matsuzawa-Ishimoto, Y. et al. Autophagy protein ATG16L1 prevents necroptosis in the intestinal epithelium. Journal of Experimental Medicine 214, 3687–3705 (2017).
- Martin, A., Emmenegger, S., Hinkelmann, K. & Thönssen, B. A viewpoint-based case-based reasoning approach utilising an enterprise architecture ontology for experience management. Enterprise Information Systems 11, 551–575 (2017).
- Markovic, B. S. et al. Bacterial Flora Play Important Roles in Acute Dextran Sulphate Sodium-Induced Colitis But Are Not Involved in Gal-3 Dependent Modulation of Colon Inflammation. Serbian Journal of Experimental and Clinical Research 18, 213–220 (2017).
- Luna-Vital, D. A., González de Mejía, E. & Loarca-Piña, G. Dietary Peptides from Phaseolus vulgaris L. Reduced AOM/DSS-Induced Colitis-Associated Colon Carcinogenesis in Balb/c Mice. Plant Foods Hum Nutr 72, 445–447 (2017).
- Li, X. et al. Myeloid-derived cullin 3 promotes STAT3 phosphorylation by inhibiting OGT expression and protects against intestinal inflammation. Journal of Experimental Medicine 214, 1093–1109 (2017).
- Khelifi, L., Soufli, I., Labsi, M. & Touil-Boukoffa, C. Immune-protective effect of echinococcosis on colitis experimental model is dependent of down regulation of TNF-α and NO production. Acta Tropica 166, 7–15 (2017).
- Katlinski, K. V. et al. Inactivation of Interferon Receptor Promotes the Establishment of Immune Privileged Tumor Microenvironment. Cancer Cell 31, 194–207 (2017).
- Hardbower, D. M. et al. EGFR-mediated macrophage activation promotes colitis-associated tumorigenesis. Oncogene 36, 3807–3819 (2017).
- Gregorio, J. D. et al. Role of glycogen synthase kinase-3β and PPAR-γ on epithelial-to-mesenchymal transition in DSS-induced colorectal fibrosis. PLOS ONE 12, e0171093 (2017).
- Fugmann, T., Sofron, A., Ritz, D., Bootz, F. & Neri, D. The MHC Class II Immunopeptidome of Lymph Nodes in Health and in Chemically Induced Colitis. J. Immunol. 198, 1357–1364 (2017).
- Fehér, Á. et al. Analysing the effect of I1 imidazoline receptor ligands on DSS-induced acute colitis in mice. Inflammopharmacol 25, 107–118 (2017).
- El‑Salhy, M., Umezawa, K., Hatlebakk, J. G. & Gilja, O. H. Abnormal differentiation of stem cells into enteroendocrine cells in rats with DSS-induced colitis. Molecular Medicine Reports 15, 2106–2112 (2017).
- El‑Salhy, M., Hatlebakk, J. G. & Gilja, O. H. Abnormalities in endocrine and immune cells are correlated in dextran‑sulfate‑sodium‑induced colitis in rats. Molecular Medicine Reports 15, 12–20 (2017).
- Do, A. et al. An HDAC6 Inhibitor Confers Protection and Selectively Inhibits B-Cell Infiltration in DSS-Induced Colitis in Mice. J Pharmacol Exp Ther 360, 140–151 (2017).
- Constante, M., Fragoso, G., Lupien-Meilleur, J., Calvé, A. & Santos, M. M. Iron Supplements Modulate Colon Microbiota Composition and Potentiate the Protective Effects of Probiotics in Dextran Sodium Sulfate-induced Colitis. Inflamm Bowel Dis 23, 753–766 (2017).
- Constante, M., Fragoso, G., Calvé, A., Samba-Mondonga, M. & Santos, M. M. Dietary Heme Induces Gut Dysbiosis, Aggravates Colitis, and Potentiates the Development of Adenomas in Mice. Front. Microbiol. 8, (2017).
- Carvajal, A. E. et al. Reelin expression is up-regulated in mice colon in response to acute colitis and provides resistance against colitis. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 1863, 462–473 (2017).
- Carvajal, A. E. et al. Reelin protects from colon pathology by maintaining the intestinal barrier integrity and repressing tumorigenic genes. Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease 1863, 2126–2134 (2017).
- Berlec, A. et al. Dextran sulphate sodium colitis in C57BL/6J mice is alleviated by Lactococcus lactis and worsened by the neutralization of Tumor necrosis Factor α. International Immunopharmacology 43, 219–226 (2017).
- Adedara, I. A., Ajayi, B. O., Awogbindin, I. O. & Farombi, E. O. Interactive effects of ethanol on ulcerative colitis and its associated testicular dysfunction in pubertal BALB/c mice. Alcohol 64, 65–75 (2017).
- Yu, C. et al. Platelet-Derived CCL5 Regulates CXC Chemokine Formation and Neutrophil Recruitment in Acute Experimental Colitis. J. Cell. Physiol. 231, 370–376 (2016).
- Vázquez-Carretero, M. D. et al. The Synaptojanins in the murine small and large intestine. J Bioenerg Biomembr 48, 569–579 (2016).
- Speca, S. et al. Novel PPARγ Modulator GED-0507-34 Levo Ameliorates Inflammation-driven Intestinal Fibrosis. Inflamm Bowel Dis 22, 279–292 (2016).
- Simovic Markovic, B. et al. Pharmacological Inhibition of Gal-3 in Mesenchymal Stem Cells Enhances Their Capacity to Promote Alternative Activation of Macrophages in Dextran Sulphate Sodium-Induced Colitis. Stem Cells International https://www.hindawi.com/journals/sci/2016/2640746/ (2016) doi:10.1155/2016/2640746.
- Simovic Markovic, B. et al. Galectin-3 Plays an Important Pro-inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages. J Crohns Colitis 10, 593–606 (2016).
- Shen, F. et al. Vinegar Treatment Prevents the Development of Murine Experimental Colitis via Inhibition of Inflammation and Apoptosis. J. Agric. Food Chem. 64, 1111–1121 (2016).
- Ohta, T. et al. Crucial roles of XCR1-expressing dendritic cells and the XCR1-XCL1 chemokine axis in intestinal immune homeostasis. Scientific Reports 6, 23505 (2016).
- O’Shea, C. J. et al. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. Journal of Nutritional Science 5, (2016).
- O’Shea, C. J. et al. The effect of algal polysaccharides laminarin and fucoidan on colonic pathology, cytokine gene expression and Enterobacteriaceae in a dextran sodium sulfate-challenged porcine model. Journal of Nutritional Science 5, (2016).
- Misiorek, J. O. et al. Keratin 8-deletion induced colitis predisposes to murine colorectal cancer enforced by the inflammasome and IL-22 pathway. Carcinogenesis 37, 777–786 (2016).
- Matthis, A. L. et al. Importance of the Evaluation of N-Acetyltransferase Enzyme Activity Prior to 5-Aminosalicylic Acid Medication for Ulcerative Colitis. Inflamm Bowel Dis 22, 1793–1802 (2016).
- Martin, J. C. et al. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunology 9, 539–549 (2016).
- Márquez-Flores, Y. K., Villegas, I., Cárdeno, A., Rosillo, M. Á. & Alarcón-de-la-Lastra, C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. The Journal of Nutritional Biochemistry 30, 143–152 (2016).
- Lee, S. et al. Arhgap17, a RhoGTPase activating protein, regulates mucosal and epithelial barrier function in the mouse colon. Scientific Reports 6, 26923 (2016).
- Kriščiukaitis, A. et al. Elaboration of Optimized Expert Knowledge Based Quantitative Features for Automatic Histological Image Evaluation. BIOMEDICAL ENGINEERING 2016 19, (2016).
- Jia, L.-G. et al. A Novel Role for TL1A/DR3 in Protection against Intestinal Injury and Infection. J. Immunol. 197, 377–386 (2016).
- Helenius, T. O., Antman, C. A., Asghar, M. N., Nyström, J. H. & Toivola, D. M. Keratins Are Altered in Intestinal Disease-Related Stress Responses. Cells 5, 35 (2016).
- Helenius, T. Structure in stress management : Keratins in intestinal stress protection. (2016).
- Heinsbroek, S. E. M. et al. miR-511-3p, embedded in the macrophage mannose receptor gene, contributes to intestinal inflammation. Mucosal Immunology 9, 960–973 (2016).
- Guada, M. et al. Cyclosporine A-loaded lipid nanoparticles in inflammatory bowel disease. International Journal of Pharmaceutics 503, 196–198 (2016).
- Goodman, W. A. et al. KLF6 contributes to myeloid cell plasticity in the pathogenesis of intestinal inflammation. Mucosal Immunology 9, 1250–1262 (2016).
- Gerling, M. et al. Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nature Communications 7, 12321 (2016).
- Farombi, E. O. et al. Dietary protocatechuic acid ameliorates dextran sulphate sodium-induced ulcerative colitis and hepatotoxicity in rats. Food Funct. 7, 913–921 (2016).
- Elmasry, A., Daba, M.-H. & El-Karef, A. Possible Effects of Moringa oleifera versus Ginger (Zingiber officinalis) on Experimental Colitis in Mice. BJMMR 16, 1–19 (2016).
- El-Salhy, M. & Umezawa, K. Treatment with novel AP-1 and NF-κB inhibitors restores the colonic endocrine cells to normal levels in rats with DSS-induced colitis. International Journal of Molecular Medicine 37, 556–564 (2016).
- El-Salhy, M. & Umezawa, K. Anti-inflammatory effects of novel AP-1 and NF-κB inhibitors in dextran-sulfate-sodium-induced colitis in rats. International Journal of Molecular Medicine 37, 1457–1464 (2016).
- el-masry, A., Daba, M.-H. & El-Karef, A. Possible Effects of Moringa oleifera versus Ginger (Zingiber officinalis) on Experimental Colitis in Mice. British Journal of Medicine and Medical Research 16, 1–19 (2016).
- Däbritz, J., Judd, L. M., Chalinor, H. V., Menheniott, T. R. & Giraud, A. S. Altered gp130 signalling ameliorates experimental colitis via myeloid cell-specific STAT3 activation and myeloid-derived suppressor cells. Sci Rep 6, 20584 (2016).
- Dolowschiak, T. et al. IFN-γ Hinders Recovery from Mucosal Inflammation during Antibiotic Therapy for Salmonella Gut Infection. Cell Host & Microbe 20, 238–249 (2016).
- Di Martino, L. et al. Protective Role for TWEAK/Fn14 in Regulating Acute Intestinal Inflammation and Colitis-Associated Tumorigenesis. Cancer Res. 76, 6533–6542 (2016).
- Di Giovangiulio, M. et al. Vagotomy Affects the Development of Oral Tolerance and Increases Susceptibility to Develop Colitis Independently of α-7 Nicotinic Receptor. Mol Med 22, 464–476 (2016).
- De Fazio, L. et al. Dietary Geraniol by Oral or Enema Administration Strongly Reduces Dysbiosis and Systemic Inflammation in Dextran Sulfate Sodium-Treated Mice. Front. Pharmacol. 7, (2016).
- Das, S. et al. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene 35, 2645–2654 (2016).
- Chng, S. H. et al. Ablating the aryl hydrocarbon receptor (AhR) in CD11c+ cells perturbs intestinal epithelium development and intestinal immunity. Scientific Reports 6, 23820 (2016).
- Brauer, R. et al. MMP-19 deficiency causes aggravation of colitis due to defects in innate immune cell function. Mucosal Immunology 9, 974–985 (2016).
- Bosma, M. et al. FNDC4 acts as an anti-inflammatory factor on macrophages and improves colitis in mice. Nature Communications 7, 11314 (2016).
- Bootz, F., Ziffels, B. & Neri, D. Antibody-Based Targeted Delivery of Interleukin-22 Promotes Rapid Clinical Recovery in Mice With DSS-Induced Colitis. Inflamm Bowel Dis 22, 2098–2105 (2016).
- Beloqui, A. et al. A comparative study of curcumin-loaded lipid-based nanocarriers in the treatment of inflammatory bowel disease. Colloids and Surfaces B: Biointerfaces 143, 327–335 (2016).
- Ahl, D. et al. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiologica 217, 300–310 (2016).
- Aden, K. et al. Epithelial IL-23R Signaling Licenses Protective IL-22 Responses in Intestinal Inflammation. Cell Reports 16, 2208–2218 (2016).
- Zwicker, S. et al. Interleukin 34: a new modulator of human and experimental inflammatory bowel disease. Clinical Science 129, 281–290 (2015).
- Zhdanov, A. V., Okkelman, I. A., Collins, F. W. J., Melgar, S. & Papkovsky, D. B. A novel effect of DMOG on cell metabolism: direct inhibition of mitochondrial function precedes HIF target gene expression. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1847, 1254–1266 (2015).
- Warfield, K. L. et al. A Novel Iminosugar UV-12 with Activity against the Diverse Viruses Influenza and Dengue (Novel Iminosugar Antiviral for Influenza and Dengue). Viruses 7, 2404–2427 (2015).
- Te Velde, A. A. et al. Effects of Dietary Plant Sterols and Stanol Esters with Low- and High-Fat Diets in Chronic and Acute Models for Experimental Colitis. Nutrients 7, 8518–8531 (2015).
- Talero, E. et al. Inhibition of chronic ulcerative colitis-associated adenocarcinoma development in mice by VSL#3. Inflamm. Bowel Dis. 21, 1027–1037 (2015).
- Spisni, E. et al. Cyclooxygenase-2 Silencing for the Treatment of Colitis: A Combined In Vivo Strategy Based on RNA Interference and Engineered Escherichia Coli. Molecular Therapy 23, 278–289 (2015).
- Soufli, I. et al. Crude extract of hydatid laminated layer from Echinococcus granulosus cyst attenuates mucosal intestinal damage and inflammatory responses in Dextran Sulfate Sodium induced colitis in mice. Journal of Inflammation 12, 19 (2015).
- Sommer, F. & Bäckhed, F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunology 8, 372–379 (2015).
- Sommer, F. & Bäckhed, F. The gut microbiota engages different signaling pathways to induce Duox2 expression in the ileum and colon epithelium. Mucosal Immunology 8, 372–379 (2015).
- Petrolis, R. et al. Digital imaging of colon tissue: method for evaluation of inflammation severity by spatial frequency features of the histological images. Diagn Pathol 10, 159 (2015).
- Moon, C. et al. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 521, 90–93 (2015).
- Hu, S. et al. The DNA Sensor AIM2 Maintains Intestinal Homeostasis via Regulation of Epithelial Antimicrobial Host Defense. Cell Reports 13, 1922–1936 (2015).
- Helenius, T. O. et al. Keratin 8 absence down-regulates colonocyte HMGCS2 and modulates colonic ketogenesis and energy metabolism. Mol. Biol. Cell 26, 2298–2310 (2015).
- Heinsbroek, S. E. M. et al. Orally delivered β-glucans aggravate dextran sulfate sodium (DSS)-induced intestinal inflammation. Nutrition Research 35, 1106–1112 (2015).
- Gouyer, V. et al. Delivery of a mucin domain enriched in cysteine residues strengthens the intestinal mucous barrier. Scientific Reports 5, 9577 (2015).
- Forte, D. et al. Human cord blood-derived platelet lysate enhances the therapeutic activity of adipose-derived mesenchymal stromal cells isolated from Crohn’s disease patients in a mouse model of colitis. Stem Cell Research & Therapy 6, 170 (2015).
- Fornasa, G. et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. Journal of Allergy and Clinical Immunology 136, 413–422 (2015).
- Elkatary, R. et al. Effect of Different Doses of Sitagliptin in Treatment of Experimentally Induced Colitis in Mice. BJPR 7, 140–151 (2015).
- Elkatary, R., Abdelrahman, K., Hassanin, A., Elmasry, A. I. & Karef, A. E. Comparative Study between Effect of Simvastatin (5 mg/Kg) and Simvastatin (50 mg/Kg) in an Early Treatment of Experimentally Induced Colitis in Mice. 1 937–947 (2015) doi:10.9734/BJMMR/2015/18314.
- Elkatary, R., Abdelrahman, K., Hassanin, A., el-masry, A. & El-Karef, A. A Comparative Study between the Effect of Simvastatin and Sitagliptin Combined and the Effect of a Large Dose of Each in an Early Treatment of Experimentally Induced Colitis in Mice. American Journal of Pharmacy and Health Research 3, (2015).
- Bootz, F., Schmid, A. S. & Neri, D. Alternatively Spliced EDA Domain of Fibronectin Is a Target for Pharmacodelivery Applications in Inflammatory Bowel Disease. Inflamm Bowel Dis 21, 1908–1917 (2015).
- Banerjee, A. et al. Umbilical cord mesenchymal stem cells modulate dextran sulfate sodium induced acute colitis in immunodeficient mice. Stem Cell Research & Therapy 6, 79 (2015).
- Asghar, M. N. et al. The Amount of Keratins Matters for Stress Protection of the Colonic Epithelium. PLOS ONE 10, e0127436 (2015).
- Ajayi, B. O., Adedara, I. A. & Farombi, E. O. Pharmacological activity of 6-gingerol in dextran sulphate sodium-induced ulcerative colitis in BALB/c mice. Phytother Res 29, 566–572 (2015).
- Yu, C. et al. Rac1 signaling regulates neutrophil-dependent tissue damage in experimental colitis. European Journal of Pharmacology 741, 90–96 (2014).
- Whelan, R. A. et al. A Transgenic Probiotic Secreting a Parasite Immunomodulator for Site-Directed Treatment of Gut Inflammation. Molecular Therapy 22, 1730–1740 (2014).
- Wang, S., Hibberd, M. L., Pettersson, S. & Lee, Y. K. Enterococcus faecalis from Healthy Infants Modulates Inflammation through MAPK Signaling Pathways. PLOS ONE 9, e97523 (2014).
- Sommer, F. et al. Altered Mucus Glycosylation in Core 1 O-Glycan-Deficient Mice Affects Microbiota Composition and Intestinal Architecture. PLOS ONE 9, e85254 (2014).
- Silva, Z. E. V. da, Lehr, H.-A. & Velin, D. In vitro and in vivo Repair Activities of Undifferentiated and Classically and Alternatively Activated Macrophages. PAT 81, 86–93 (2014).
- Shaker, M. E., Ashamallah, S. A. & Houssen, M. E. Celastrol ameliorates murine colitis via modulating oxidative stress, inflammatory cytokines and intestinal homeostasis. Chemico-Biological Interactions 210, 26–33 (2014).
- Ramonaite, R. et al. Protective action of NADPH oxidase inhibitors and role of NADPH oxidase in pathogenesis of colon inflammation in mice. World J Gastroenterol 20, 12533–12541 (2014).
- Pineton de Chambrun, G. et al. Aluminum enhances inflammation and decreases mucosal healing in experimental colitis in mice. Mucosal Immunology 7, 589–601 (2014).
- Maillard, M. H. et al. Toll-interacting Protein Modulates Colitis Susceptibility in Mice. Inflamm Bowel Dis 20, 660–670 (2014).
- Kernbauer, E., Ding, Y. & Cadwell, K. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516, 94–98 (2014).
- Jädert, C., Phillipson, M., Holm, L., Lundberg, J. O. & Borniquel, S. Preventive and therapeutic effects of nitrite supplementation in experimental inflammatory bowel disease. Redox Biology 2, 73–81 (2014).
- Judd, L. M. et al. TFF2 deficiency exacerbates weight loss and alters immune cell and cytokine profiles in DSS colitis, and this cannot be rescued by wild-type bone marrow. American Journal of Physiology-Gastrointestinal and Liver Physiology 308, G12–G24 (2014).
- Jakobsson, T. et al. The oxysterol receptor LXRβ protects against DSS- and TNBS-induced colitis in mice. Mucosal Immunology 7, 1416–1428 (2014).
- Gerling, M. et al. Real-Time Assessment of Tissue Hypoxia In Vivo with Combined Photoacoustics and High-Frequency Ultrasound. Theranostics 4, 604–613 (2014).
- Felgines, C., Fraisse, D., Besson, C., Vasson, M.-P. & Texier, O. Bioavailability of lemon verbena (Aloysia triphylla) polyphenols in rats: impact of colonic inflammation. British Journal of Nutrition 111, 1773–1781 (2014).
- Ding, S., Blue, R. E., Morgan, D. R. & Lund, P. K. Comparison of Multiple Enzyme Activatable Near-Infrared Fluorescent Molecular Probes for Detection and Quantification of Inflammation in Murine Colitis Models. Inflamm Bowel Dis 20, 363–377 (2014).
- De Fazio, L. et al. Longitudinal analysis of inflammation and microbiota dynamics in a model of mild chronic dextran sulfate sodium-induced colitis in mice. World J Gastroenterol 20, 2051–2061 (2014).
- Canavan, M. et al. Activation of liver X receptor suppresses the production of the IL-12 family of cytokines by blocking nuclear translocation of NF-κBp50. Innate Immun 20, 675–687 (2014).
- Bär, F. et al. Carboxypeptidase E Modulates Intestinal Immune Homeostasis and Protects against Experimental Colitis in Mice. PLOS ONE 9, e102347 (2014).
- Beloqui, A. et al. pH-sensitive nanoparticles for colonic delivery of curcumin in inflammatory bowel disease. International Journal of Pharmaceutics 473, 203–212 (2014).
- Asghar, M. N. et al. In vivo imaging of reactive oxygen and nitrogen species in murine colitis. Inflamm. Bowel Dis. 20, 1435–1447 (2014).
- Alhouayek, M. et al. N-Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. The FASEB Journal 29, 650–661 (2014).
- Wagner, A. E. et al. DSS-induced acute colitis in C57BL/6 mice is mitigated by sulforaphane pre-treatment. The Journal of Nutritional Biochemistry 24, 2085–2091 (2013).
- Tourteau, A. et al. 3-Carboxamido-5-aryl-isoxazoles as new CB2 agonists for the treatment of colitis. Bioorganic & Medicinal Chemistry 21, 5383–5394 (2013).
- Thaker, A. I. et al. IDO1 Metabolites Activate β-catenin Signaling to Promote Cancer Cell Proliferation and Colon Tumorigenesis in Mice. Gastroenterology 145, 416-425.e4 (2013).
- Sina, C. et al. Extracellular cathepsin K exerts antimicrobial activity and is protective against chronic intestinal inflammation in mice. Gut 62, 520–530 (2013).
- Schreiber, O. et al. iNOS-Dependent Increase in Colonic Mucus Thickness in DSS-Colitic Rats. PLOS ONE 8, e71843 (2013).
- Sann, H., Erichsen, J. von, Hessmann, M., Pahl, A. & Hoffmeyer, A. Efficacy of drugs used in the treatment of IBD and combinations thereof in acute DSS-induced colitis in mice. Life Sciences 92, 708–718 (2013).
- Ranganathan, P., Jayakumar, C., Santhakumar, M. & Ramesh, G. Netrin-1 regulates colon-kidney cross talk through suppression of IL-6 function in a mouse model of DSS-colitis. Am J Physiol Renal Physiol 304, F1187–F1197 (2013).
- Ranganathan, P., Jayakumar, C., Santhakumar, M. & Ramesh, G. Netrin-1 regulates colon-kidney cross talk through suppression of IL-6 function in a mouse model of DSS-colitis. American Journal of Physiology-Renal Physiology 304, F1187–F1197 (2013).
- O’Carroll, C. et al. Bcl-3 deficiency protects against dextran-sodium sulphate-induced colitis in the mouse. Clinical & Experimental Immunology 173, 332–342 (2013).
- Montbarbon, M. et al. Colonic Inflammation in Mice Is Improved by Cigarette Smoke through iNKT Cells Recruitment. PLOS ONE 8, e62208 (2013).
- McCarthy, J. et al. Gene silencing of TNF-alpha in a murine model of acute colitis using a modified cyclodextrin delivery system. Journal of Controlled Release 168, 28–34 (2013).
- Konieczna, P. et al. Immunomodulation by Bifidobacterium infantis 35624 in the Murine Lamina Propria Requires Retinoic Acid-Dependent and Independent Mechanisms. PLOS ONE 8, e62617 (2013).
- Kleiveland, C. R. et al. The Noncommensal Bacterium Methylococcus capsulatus (Bath) Ameliorates Dextran Sulfate (Sodium Salt)-Induced Ulcerative Colitis by Influencing Mechanisms Essential for Maintenance of the Colonic Barrier Function. Appl. Environ. Microbiol. 79, 48–56 (2013).
- Hall, L. J. et al. Natural killer cells protect mice from DSS-induced colitis by regulating neutrophil function via the NKG2A receptor. Mucosal Immunology 6, 1016–1026 (2013).
- Guzman, J. R. et al. Oxymatrine Prevents NF-κB Nuclear Translocation And Ameliorates Acute Intestinal Inflammation. Scientific Reports 3, 1629 (2013).
- Grimstad, T. et al. A salmon peptide diet alleviates experimental colitis as compared with fish oil. Journal of Nutritional Science 2, (2013).
- Gillberg, L. et al. Effective treatment of mouse experimental colitis by alpha 2 integrin antibody: comparison with alpha 4 antibody and conventional therapy. Acta Physiologica 207, 326–336 (2013).
- Couturier-Maillard, A. et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest 123, (2013).
- Bär, F. et al. Mitochondrial Gene Polymorphisms That Protect Mice From Colitis. Gastroenterology 145, 1055-1063.e3 (2013).
- Brun, P. et al. Peroxisome proliferator-activated receptor-γ mediates the anti-inflammatory effect of 3-hydroxy-4-pyridinecarboxylic acid derivatives: Synthesis and biological evaluation. European Journal of Medicinal Chemistry 62, 486–497 (2013).
- Brounais-Le Royer, B. et al. Effects of an Interleukin-15 Antagonist on Systemic and Skeletal Alterations in Mice with DSS-Induced Colitis. The American Journal of Pathology 182, 2155–2167 (2013).
- Bjørndal, B. et al. Tetradecylthioacetic Acid Attenuates Inflammation and Has Antioxidative Potential During Experimental Colitis in Rats. Dig Dis Sci 58, 97–106 (2013).
- Beloqui, A. et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. International Journal of Pharmaceutics 454, 775–783 (2013).
- Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
- Ackermann, M., Tsuda, A., Secomb, T. W., Mentzer, S. J. & Konerding, M. A. Intussusceptive remodeling of vascular branch angles in chemically-induced murine colitis. Microvascular Research 87, 75–82 (2013).
- Øines, E., Murison, R., Mrdalj, J., Grønli, J. & Milde, A. M. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiology & Behavior 105, 1058–1066 (2012).
- Xu, C., Shen, Y., Littman, D. R., Dustin, M. L. & Velázquez, P. Visualization of mucosal homeostasis via single- and multiphoton intravital fluorescence microscopy. Journal of Leukocyte Biology 92, 413–419 (2012).
- Wadie, W. et al. STW 5 is effective in dextran sulfate sodium-induced colitis in rats. Int J Colorectal Dis 27, 1445–1453 (2012).
- Thaker, A. I., Shaker, A., Rao, M. S. & Ciorba, M. A. Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS). J Vis Exp (2012) doi:10.3791/4100.
- Thaker, A. I., Shaker, A., Rao, M. S. & Ciorba, M. A. Modeling Colitis-Associated Cancer with Azoxymethane (AOM) and Dextran Sulfate Sodium (DSS). JoVE (Journal of Visualized Experiments) e4100 (2012) doi:10.3791/4100.
- Takiguchi, H. et al. Reduced production of polymeric immunoglobulin receptor in murine dextran sodium sulfate-induced colitis. Journal of Oral Science 54, 23–32 (2012).
- Spadoni, I., Iliev, I. D., Rossi, G. & Rescigno, M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunology 5, 184–193 (2012).
- Rang, S. et al. Lactobacillus reuteri maintains a functional mucosal barrier during DSS treatment despite mucus layer dysfunction. ERA https://era.library.ualberta.ca/items/fb7a704e-2931-467e-8efd-c60fa8828b6e (2012) doi:10.7939/R3DJ58W4W.
- Philipp, E. E. R. et al. Massively Parallel RNA Sequencing Identifies a Complex Immune Gene Repertoire in the lophotrochozoan Mytilus edulis. PLOS ONE 7, e33091 (2012).
- Lied, G. A. et al. Increased wall thickness using ultrasonography is associated with inflammation in an animal model of experimental colitis. Clin Exp Gastroenterol 5, 195–201 (2012).
- Heinsbroek, S. E. et al. Genetic deletion of dectin-1 does not affect the course of murine experimental colitis. BMC Gastroenterol 12, 33 (2012).
- Grimstad, T. et al. Dietary supplementation of krill oil attenuates inflammation and oxidative stress in experimental ulcerative colitis in rats. Scandinavian Journal of Gastroenterology 47, 49–58 (2012).
- Gottfries, J., Melgar, S. & Michaëlsson, E. Modelling of Mouse Experimental Colitis by Global Property Screens: A Holistic Approach to Assess Drug Effects in Inflammatory Bowel Disease. PLOS ONE 7, e30005 (2012).
- Ding, S. et al. Mucosal Healing and Fibrosis after Acute or Chronic Inflammation in Wild Type FVB-N Mice and C57BL6 Procollagen α1(I)-Promoter-GFP Reporter Mice. PLOS ONE 7, e42568 (2012).
- Dicksved, J. et al. Lactobacillus reuteri Maintains a Functional Mucosal Barrier during DSS Treatment Despite Mucus Layer Dysfunction. PLOS ONE 7, e46399 (2012).
- DeBoer, M. D., Steinman, J. & Li, Y. Partial normalization of pubertal timing in female mice with DSS colitis treated with anti-TNF-α antibody. J Gastroenterol 47, 647–654 (2012).
- de Jong, J. H. et al. Fusion of intestinal epithelial cells with bone marrow derived cells is dispensable for tissue homeostasis. Scientific Reports 2, 271 (2012).
- Borniquel, S., Jädert, C. & Lundberg, J. O. Dietary Conjugated Linoleic Acid Activates PPARγ and the Intestinal Trefoil Factor in SW480 Cells and Mice with Dextran Sulfate Sodium-Induced Colitis. J Nutr 142, 2135–2140 (2012).
- Villegas, I., Sánchez‐Fidalgo, S. & Lastra, C. A. de la. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Molecular Nutrition & Food Research 55, 259–267 (2011).
- Rahman, A. et al. Chronic colitis induces expression of β-defensins in murine intestinal epithelial cells. Clinical & Experimental Immunology 163, 123–130 (2011).
- Queiroz, K. C. S. et al. Tissue Factor-Dependent Chemokine Production Aggravates Experimental Colitis. Mol Med 17, 1119–1126 (2011).
- Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. PNAS 108, 9601–9606 (2011).
- Lenoir, L. et al. Lemon Verbena Infusion Consumption Attenuates Oxidative Stress in Dextran Sulfate Sodium-Induced Colitis in the Rat. Dig Dis Sci 56, 3534–3545 (2011).
- Hall, L. J. et al. Induction and Activation of Adaptive Immune Populations During Acute and Chronic Phases of a Murine Model of Experimental Colitis. Dig Dis Sci 56, 79–89 (2011).
- Deboer, M. D. & Li, Y. Puberty Is Delayed in Male Mice With Dextran Sodium Sulfate Colitis Out of Proportion to Changes in Food Intake, Body Weight, and Serum Levels of Leptin. Pediatric Research 69, 34–39 (2011).
- Cançado, G. G. L. et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. Inflamm Bowel Dis 17, 2275–2286 (2011).
- Banerjee, S. et al. Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. American Journal of Physiology-Gastrointestinal and Liver Physiology 300, G273–G282 (2011).
- van Dop, W. A. et al. The absence of functional PI3Kγ prevents leukocyte recruitment and ameliorates DSS-induced colitis in mice. Immunology Letters 131, 33–39 (2010).
- Snoek, S. A. et al. Selective α7 nicotinic acetylcholine receptor agonists worsen disease in experimental colitis. British Journal of Pharmacology 160, 322–333 (2010).
- Sina, C. et al. Ablation of gly96/immediate early gene-X1 (gly96/iex-1) aggravates DSS-induced colitis in mice: Role for gly96/iex-1 in the regulation of NF-κB. Inflamm Bowel Dis 16, 320–331 (2010).
- Sánchez-Fidalgo, S. et al. Extra-virgin olive oil-enriched diet modulates DSS-colitis-associated colon carcinogenesis in mice. Clinical Nutrition 29, 663–673 (2010).
- Petersson, J. et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 300, G327–G333 (2010).
- Murphy, C. T. et al. Technical Advance: Function and efficacy of an α4-integrin antagonist using bioluminescence imaging to detect leukocyte trafficking in murine experimental colitis. Journal of Leukocyte Biology 88, 1271–1278 (2010).
- Murphy, C. T. et al. Technical Advance: Function and efficacy of an α4-integrin antagonist using bioluminescence imaging to detect leukocyte trafficking in murine experimental colitis. Journal of Leukocyte Biology 88, 1271–1278 (2010).
- Murphy, C. T. et al. Use of bioluminescence imaging to track neutrophil migration and its inhibition in experimental colitis. Clinical & Experimental Immunology 162, 188–196 (2010).
- Konerding, M. A. et al. Inflammation-Induced Intussusceptive Angiogenesis in Murine Colitis. The Anatomical Record 293, 849–857 (2010).
- Johansson, M. E. V. et al. Bacteria Penetrate the Inner Mucus Layer before Inflammation in the Dextran Sulfate Colitis Model. PLOS ONE 5, e12238 (2010).
- Edelson, B. T. et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. Journal of Experimental Medicine 207, 823–836 (2010).
- DeBoer, M. D., Li, Y. & Cohn, S. Colitis causes delay in puberty in female mice out of proportion to changes in leptin and corticosterone. J Gastroenterol 45, 277–284 (2010).
- Ciorba, M. A. et al. Induction of IDO-1 by Immunostimulatory DNA Limits Severity of Experimental Colitis. The Journal of Immunology 184, 3907–3916 (2010).
- Cadwell, K. et al. Virus-Plus-Susceptibility Gene Interaction Determines Crohn’s Disease Gene Atg16L1 Phenotypes in Intestine. Cell 141, 1135–1145 (2010).
- Borniquel, S., Jansson, E. Å., Cole, M. P., Freeman, B. A. & Lundberg, J. O. Nitrated oleic acid up-regulates PPARγ and attenuates experimental inflammatory bowel disease. Free Radical Biology and Medicine 48, 499–505 (2010).
- Bernasconi, E. et al. Granulocyte-macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm Bowel Dis 16, 428–441 (2010).
- Zheng, L., Riehl, T. E. & Stenson, W. F. Regulation of Colonic Epithelial Repair in Mice by Toll-Like Receptors and Hyaluronic Acid. Gastroenterology 137, 2041–2051 (2009).
- Turhan, A. et al. Vascular Microarchitecture of Murine Colitis-Associated Lymphoid Angiogenesis. The Anatomical Record 292, 621–632 (2009).
- Tsuda, A. et al. Bimodal Oscillation Frequencies of Blood Flow in the Inflammatory Colon Microcirculation. The Anatomical Record 292, 65–72 (2009).
- Sina, C. et al. G Protein-Coupled Receptor 43 Is Essential for Neutrophil Recruitment during Intestinal Inflammation. The Journal of Immunology 183, 7514–7522 (2009).
- Schreiber, O. et al. Lactobacillus reuteri prevents colitis by reducing P-selectin-associated leukocyte- and platelet-endothelial cell interactions. American Journal of Physiology-Gastrointestinal and Liver Physiology 296, G534–G542 (2009).
- Miele, L. F. et al. Blood Flow Patterns Spatially Associated with Platelet Aggregates in Murine Colitis. The Anatomical Record 292, 1143–1153 (2009).
- Lång, P., Lange, S., Delbro, D. & Andersson, G. Induction and cellular expression of tartrate resistant acid phosphatase during dextran sodium sulphate induced colitis in rats. Histochem Cell Biol 132, 599 (2009).
- Iliev, I. D., Mileti, E., Matteoli, G., Chieppa, M. & Rescigno, M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunology 2, 340–350 (2009).
- Huang, T.-Y. et al. Minocycline attenuates experimental colitis in mice by blocking expression of inducible nitric oxide synthase and matrix metalloproteinases. Toxicology and Applied Pharmacology 237, 69–82 (2009).
- Hansen, J. J., Holt, L. & Sartor, B. R. Gene Expression Patterns in Experimental Colitis in IL-10-Deficient Mice. Inflamm Bowel Dis 15, 890–899 (2009).
- Davies, P. S., Powell, A. E., Swain, J. R. & Wong, M. H. Inflammation and Proliferation Act Together to Mediate Intestinal Cell Fusion. PLOS ONE 4, e6530 (2009).
- Canevari, M. et al. Poly(ethylene glycol)-mesalazine conjugate for colon specific delivery. International Journal of Pharmaceutics 368, 171–177 (2009).
- Banerjee, S. et al. MEP1A allele for meprin A metalloprotease is a susceptibility gene for inflammatory bowel disease. Mucosal Immunol 2, 220–231 (2009).
- Voltan, S. et al. Lactobacillus crispatus M247-Derived H2O2 Acts as a Signal Transducing Molecule Activating Peroxisome Proliferator Activated Receptor-γ in the Intestinal Mucosa. Gastroenterology 135, 1216–1227 (2008).
- Turhan, A. et al. Bridging Mucosal Vessels Associated with Rhythmically Oscillating Blood Flow in Murine Colitis. The Anatomical Record 291, 74–82 (2008).
- Ramakers, J. D., Mensink, R. P., Verstege, M. I., Velde, A. A. te & Plat, J. An arachidonic acid-enriched diet does not result in more colonic inflammation as compared with fish oil- or oleic acid-enriched diets in mice with experimental colitis. British Journal of Nutrition 100, 347–354 (2008).
- Melgar, S. et al. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. International Immunopharmacology 8, 836–844 (2008).
- Melgar, S., Engström, K., Jägervall, Å. & Martinez, V. Psychological stress reactivates dextran sulfate sodium-induced chronic colitis in mice. Stress 11, 348–362 (2008).
- Matters, G. L. et al. The Opioid Antagonist Naltrexone Improves Murine Inflammatory Bowel Disease. Journal of Immunotoxicology 5, 179–187 (2008).
- Li, H. et al. Intestinal, adipose, and liver inflammation in diet-induced obese mice. Metabolism 57, 1704–1710 (2008).
- Karlsson, A. et al. Dextran sulphate sodium induces acute colitis and alters hepatic function in hamsters. International Immunopharmacology 8, 20–27 (2008).
- Fritsch Fredin, M. et al. The application and relevance of ex vivo culture systems for assessment of IBD treatment in murine models of colitis. Pharmacological Research 58, 222–231 (2008).
- Spek, A. C., Kate, F. J. W. ten & Velde, A. A. te. Factor V Leiden and the etiology of inflammatory bowel disease. Thromb Haemost 98, 670–673 (2007).
- Rigby, R. J., Simmons, J. G., Greenhalgh, C. J., Alexander, W. S. & Lund, P. K. Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene 26, 4833–4841 (2007).
- Ramakers, J. D. et al. The PPARγ Agonist Rosiglitazone Impairs Colonic Inflammation in Mice with Experimental Colitis. J Clin Immunol 27, 275–283 (2007).
- Petersson, J. et al. eNOS involved in colitis-induced mucosal blood flow increase. American Journal of Physiology-Gastrointestinal and Liver Physiology 293, G1281–G1287 (2007).
- Nysœter, G. et al. Effect of live Salmonella Ty21a in Dextran Sulfate Sodium-induced Colitis. Drug Target�Insights 2, DTI.S220 (2007).
- Melgar, S., Gillberg, P.-G., Hockings, P. D. & Olsson, L. E. High-throughput magnetic resonance imaging in murine colonic inflammation. Biochemical and Biophysical Research Communications 355, 1102–1107 (2007).
- Melgar, S. et al. Mice with experimental colitis show an altered metabolism with decreased metabolic rate. American Journal of Physiology-Gastrointestinal and Liver Physiology 292, G165–G172 (2007).
- Holma, R., Salmenperä, P., Virtanen, I., Vapaatalo, H. & Korpela, R. Prophylactic potential of montelukast against mild colitis induced by dextran sulphate sodium in rats. J. Physiol. Pharmacol. 58, 455–467 (2007).
- Brown, S. L. et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest 117, 258–269 (2007).
- Arslan, G. et al. No Protection against DSS-induced Colitis by Short-term Pretreatment with Seal or Fish Oils in Rats. Integr Med�Insights 2, 117863370700200000 (2007).
- Van der Sluis, M. et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 131, 117–129 (2006).
- Lundberg, S., Lindholm, J., Lindbom, L., Hellström, P. M. & Werr, J. Integrin α2β1 Regulates Neutrophil Recruitment and Inflammatory Activity in Experimental Colitis in Mice. Inflamm Bowel Dis 12, 172–177 (2006).
- Larsson, M. H., Rapp, L. & Lindström, E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterology & Motility 18, 144–152 (2006).
- Larsson, A. E. et al. Magnetic resonance imaging of experimental mouse colitis and association with inflammatory activity. Inflamm Bowel Dis 12, 478–485 (2006).
- Giannakis, M. et al. Molecular Properties of Adult Mouse Gastric and Intestinal Epithelial Progenitors in Their Niches. J. Biol. Chem. 281, 11292–11300 (2006).
- Chidlow, J. H. et al. Differential Angiogenic Regulation of Experimental Colitis. The American Journal of Pathology 169, 2014–2030 (2006).
- Brand, S. et al. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. American Journal of Physiology-Gastrointestinal and Liver Physiology 290, G827–G838 (2006).
- Sund, M. et al. Reduced susceptibility to dextran sulphate sodium-induced colitis in the interleukin-2 heterozygous (IL-2+/–) mouse. Immunology 114, 554–564 (2005).
- Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I. & Stappenbeck, T. S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. PNAS 102, 99–104 (2005).
- Milde, A. M., Arslan, G., Overmier, J. B., Berstad, A. & Murison, R. An acute stressor enhances sensitivity to a chemical irritant and increases51CrEDTA permeability of the colon in adult rats. Integr. psych. behav. 40, 35–44 (2005).
- Melgar, S., Karlsson, A. & Michaëlsson, E. Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology 288, G1328–G1338 (2005).
- Elrod, J. W. et al. DSS-Induced Colitis Is Exacerbated in STAT-6 Knockout Mice. Inflamm Bowel Dis 11, 883–889 (2005).
- Castagliuolo, I. et al. Beneficial effect of auto-aggregating Lactobacillus crispatus on experimentally induced colitis in mice. FEMS Immunol Med Microbiol 43, 197–204 (2005).
- Brun, P. et al. Neuropeptide neurotensin stimulates intestinal wound healing following chronic intestinal inflammation. American Journal of Physiology-Gastrointestinal and Liver Physiology 288, G621–G629 (2005).
- Bennink, R. J. et al. Dedicated Pinhole SPECT of Intestinal Neutrophil Recruitment in a Mouse Model of Dextran Sulfate Sodium–Induced Colitis. J Nucl Med 46, 526–531 (2005).
- Milde, A. M., Enger, Ø. & Murison, R. The effects of postnatal maternal separation on stress responsivity and experimentally induced colitis in adult rats. Physiology & Behavior 81, 71–84 (2004).
- Leemans, J. C. et al. The Epidermal Growth Factor-Seven Transmembrane (EGF-TM7) Receptor CD97 Is Required for Neutrophil Migration and Host Defense. The Journal of Immunology 172, 1125–1131 (2004).
- Edalat, M., Mannervik, B. & Axelsson, L.-G. Selective expression of detoxifying glutathione transferases in mouse colon: effect of experimental colitis and the presence of bacteria. Histochem Cell Biol 122, 151–159 (2004).
- Bene, L. et al. Partial Protection against Dextran Sodium Sulphate Induced Colitis in Histamine-Deficient, Histidine Decarboxylase Knockout Mice. Journal of Pediatric Gastroenterology and Nutrition 39, 171 (2004).
- Sasaki, M. et al. The 3-Hydroxy-3-methylglutaryl-CoA Reductase Inhibitor Pravastatin Reduces Disease Activity and Inflammation in Dextran-Sulfate Induced Colitis. J Pharmacol Exp Ther 305, 78–85 (2003).
- Sasaki, M. et al. Increased disease activity in eNOS-deficient mice in experimental colitis. Free Radical Biology and Medicine 35, 1679–1687 (2003).
- Milde, A. M., Sundberg, H., RØseth, A. G. & Murison, R. Proactive Sensitizing Effects of Acute Stress on Acoustic Startle Responses and Experimentally Induced Colitis in Rats: Relationship to Corticosterone. Stress 6, 49–57 (2003).
- Milde, A. M., Sundberg, H., RØseth, A. G. & Murison, R. Proactive Sensitizing Effects of Acute Stress on Acoustic Startle Responses and Experimentally Induced Colitis in Rats: Relationship to Corticosterone. Stress 6, 49–57 (2003).
- Håversen, L. A., Baltzer, L., Dolphin, G., Hanson, L. Å. & Mattsby‐Baltzer, I. Anti-Inflammatory Activities of Human Lactoferrin in Acute Dextran Sulphate-Induced Colitis in Mice. Scandinavian Journal of Immunology 57, 2–10 (2003).
- Börjesson, L. & Delbro, D. S. Neurogenic and non-neurogenic mechanisms in response of rat distal colon muscle to dextran sulphate sodium treatment. Autonomic Neuroscience 107, 74–80 (2003).
- Spiik, A.-K. et al. Abrogated lymphocyte infiltration and lowered CD14 in dextran sulfate induced colitis in mice treated with p65 antisense oligonucleotides. Int J Colorectal Dis 17, 223–232 (2002).
- Renes, I. B. et al. Distinct epithelial responses in experimental colitis: implications for ion uptake and mucosal protection. American Journal of Physiology-Gastrointestinal and Liver Physiology 283, G169–G179 (2002).
- Milde, A. M. & Murison, R. A study of the effets of restraint stress on colitis induced by dextran sulphate sodium in singly housed rats. Integrative Physiological & Behavioral Science 37, 140–150 (2002).
- Castagliuolo, I. et al. Protective effects of neurokinin-1 receptor during colitis in mice: role of the epidermal growth factor receptor. British Journal of Pharmacology 136, 271–279 (2002).
- Williams, K. L. et al. Enhanced survival and mucosal repair after dextran sodium sulfate–induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology 120, 925–937 (2001).
- Stevceva, L., Pavli, P., Husband, A., Ramsay, A. & Doe, W. F. Dextran sulphate sodium-induced colitis is ameliorated in interleukin 4 deficient mice. Genes Immun 2, 309–316 (2001).
- Börjesson, L., Aldenborg, F. & Delbro, D. S. Functional effects of dextran sulphate sodium (DSS) treatment on the longitudinal muscle of rat distal colon. Journal of Autonomic Pharmacology 21, 121–129 (2001).
- Stevceva, L. et al. Eosinophilia is attenuated in experimental colitis induced in IL-5 deficient mice. Genes Immun 1, 213–218 (2000).
- Morteau, O. et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J Clin Invest 105, 469–478 (2000).
- Stevceva, L., Pavli, P., Buffinton, G., Wozniak, A. & Doe, W. Dextran sodium sulphate-induced colitis activity varies with mouse strain but develops in lipopolysaccharide-unresponsive mice. Journal of Gastroenterology and Hepatology 14, 54–60 (1999).
- Mähler, M. et al. Genetic Analysis of Susceptibility to Dextran Sulfate Sodium-Induced Colitis in Mice. Genomics 55, 147–156 (1999).
- Blackburn, A. C., Doe, W. F. & Buffinton, G. D. Protein carbonyl formation on mucosal proteins in vitro and in dextran sulfate-induced colitis. Free Radical Biology and Medicine 27, 262–270 (1999).
- Mähler, M. et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology 274, G544–G551 (1998).
- Dieleman, L. A. et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 114, 385–391 (1998).
- Blackburn, A. C., Doe, W. F. & Buffinton, G. D. Salicylate Hydroxylation as an Indicator of Hydroxyl Radical Generation in Dextran Sulfate-Induced Colitis. Free Radical Biology and Medicine 25, 305–313 (1998).
- Axelsson, L.-G., Landström, E. & Bylund‐Fellenius, A.-C. Experimental colitis induced by dextran sulphate sodium in mice: beneficial effects of sulphasalazine and olsalazine. Alimentary Pharmacology & Therapeutics 12, 925–934 (1998).
- Blackburn, A. C., Doe, W. F. & Buffinton, G. D. Colonic antioxidant status in dextran sulfate-induced colitis in mice. Inflammatory Bowel Diseases 3, 198–203 (1997).
- Lange, S., Delbro, D. S., Jennische, E. & Mattsby‐Baltzer, I. The role of the Lps gene in experimental ulcerative colitis in mice. APMIS 104, 823–833 (1996).
- Axelsson, L.-G., Midtvedt, T. & Bylund-Fellenius, A.-C. The Role of Intestinal Bacteria, Bacterial Translocation and Endotoxin in Dextran Sodium Sulphate-Induced Colitis in the Mouse. Microbial Ecology in Health and Disease 9, 225–237 (1996).
- Axelsson, L.-G., Landström, E., Goldschmidt, T. J., Grönberg, A. & Bylund-Fellenius, A.-C. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: Effects in CD4+-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res 45, 181–191 (1996).
- Dieleman, L. A. et al. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology 107, 1643–1652 (1994).


